What is microflow LC?
What are the differences between micro-scale and conventional LC?
Microflow (a.k.a. microscale) LC has been widely applied to proteomic research for years. Recently, as microflow LC-MS systems continue to evolve, more and more laboratories in various fields are weighing the implications of applying this small-is-beautiful technology.
In this and my next two blog posts, I will discuss how the benefits stack up against the tradeoffs while moving to a microflow platform and what laboratories across a diverse range of disciplines can do to tilt the reward and risk ratio to their favor.
What exactly is microflow LC?
Compared to the conventional flow, the microflow approach downsizes two key variables:
- The inner diameter (ID) of the fluidic channel inside the separation device, for example, the LC column;
- The volume of fluid flowing through the columns in a given time.
Although there’s no universal definition of microflow, it typically refers to a flow rate ranging from 1 to 100 µL/min or the use of columns with an ID between 0.1 and 1.0 mm.
What are the benefits of microflow LC?
With a small ID column, LC systems require a lower fluidic flow to perform a chromatographic separation, which consumes less solvent and, therefore, it makes the microflow LC an environment friendly or greener technology. As a result, LC-MS becomes a meaner, leaner, and greener instrumental technique.
Of course, this is NOT the only reason that many laboratories insist on using this platform. Most importantly, this scaled-down approach provides the ultimate edge, the sensitivity gain over its high flow rate counterparts.
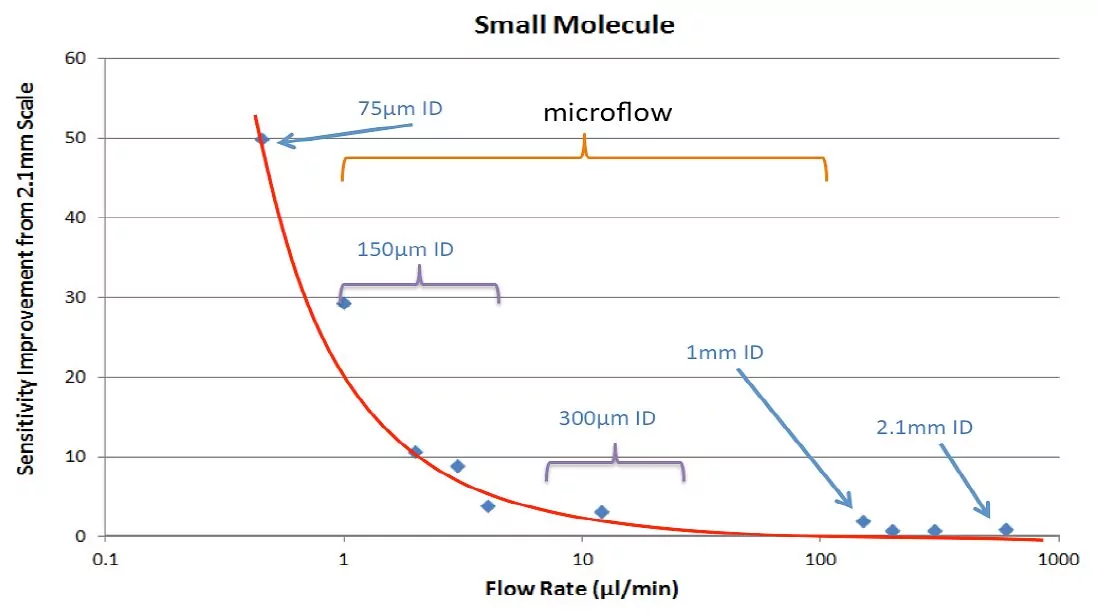
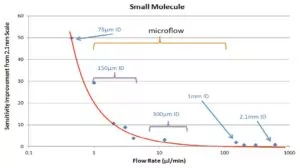
The graph in Figure 1 shows that for some analytes, column IDs at the lower end of the microscale range can boost sensitivity in a big way. There is a huge sensitivity boost once the column ID drops to 150 µm. For certain peptides, the sensitivity gain can go up 40 fold.
The increase in MS signal intensity has nothing to do with concentration of the analyte on a column. These sensitivity gains stem largely from improving sampling efficiency and reducing matrix effects (matrix effects will be discussed in my next post).
Let’s take a look at how microflow LC improves sampling efficiency…
At flow rates >100 µL/min, a significant portion of sensitivity is lost due to limited sampling efficiency. An electrospray plume generated from conventional LC flow can be quite broad and divergent (Figure 2). The inlet to a mass spectrometer has the ability to sample only a portion of the plume. In order to maximize the sampling efficiency, the most common approach is to position the MS inlet orthogonally to the electrospray probe to sample the edges of the electrospray plume where fine droplets are present.
As the solvent flow rate is reduced, the electrospray plume decreases in size and becomes more convergent as seen in Figure 2 on the left. This allows the inlet of the mass spectrometer to become more efficient to capture a greater percentage of the plume. This results in an increasing signal.

A good analogy is a child drinking from a water fountain. As you can see from the picture on the left below, the child does a great job drinking a small amount of water when it’s a fine flow from a small outlet. She may not be perfect and some water may get on her dress, but overall she does a good job and the intended outcome is accomplished. If we ask a child to try and drink from a sprinkler, like you can see in the picture on the right below … well, let’s just say this task becomes much less efficient and more difficult to accomplish.
Another significant performance advantage is microflow’s potential to simplify analysis by expanding the dynamic range of analyte measurement. For some analytes, microflow extends the upper limit as well as the lower limit of detection, eliminating the need to run multiple experiments to determine the concentrations of both high- and low-abundance compounds that occur together in complex mixtures.
By using smaller flow rate coupled with higher sensitivity, microfluidic experiments enable the do-more-with-less request from users who need to conserve precious sample, reduce the costs of solvent, or minimize the environmental impacts of using toxic reagents.
The benefits of microflow LC-MS provides laboratory scientists with a valuable measure between nanoscale and macroscale platforms. Microflow LC-MS is not a new technology and because of its sensitivity gains, not only has it been used in proteomics research, it is now applied in biomarker discovery, bioanalysis, and environmental analysis.
Now that we have reviewed what we consider to be good aspects of microflow LC-MS, what are considered to be bad aspects of microflow LC/MS? Is everything about microflow wonderful? Is it true that microflow can be painstaking? Does it require highly skilled users to perform the work? Is it possible to use it for routine analysis?
In my future post, I will answer these questions as well as discuss the technical breakthrough of the microflow LC-MS that has occurred in recent years.
Related reading:
- Evolution in miniaturized column liquid chromatography instrumentation and applications: An overview
- A Review of Nanoelectrospray Ionization Applications for Drug Metabolism and Pharmacokinetics
- Enhancing Mass Spectrometry Sensitivity by Reducing Chromatographic Flow Rates with ionKey/MS
Related posts:
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)




