Waters GlycoWorks RapiFluor-MS N-Glycan Kit Improves Reproducibility and Speed of N-Glycan Analysis Through Innovation and Automation
Glycosylation is an important post-translational modification that can impact the safety and efficacy of protein therapeutics and is therefore one of the most analyzed product attributes during development and quality control. In released N-glycan analysis, glycans are cleaved from proteins via enzymatic digestion and are chemically derivatized for relative quantification by liquid chromatography coupled to fluorescence and mass spectrometry, or capillary electrophoresis with laser-induced fluorescence detection.
The inherent challenges of N-glycan analysis have pushed the industry to improve upon best practices. Waters introduction of the GlycoWorks RapiFluor-MS N-Glycan Kit features a novel reagent and platform method for N-Glycan analysis of biotherapeutics, reducing sample preparation time from days to one hour.
GlycoWorks includes an optically active and mass spectrometry friendly reagent, RapiFluor-MS, providing industry leading sensitivity for all released N-glycan species. Complemented by the BioAccord LC-MS System, this biotherapeutic analysis solution delivers high-resolution results in a compact, compliant-ready format.
Released N-glycan sample preparation procedures involve multiple steps including enzymatic deglycosylation, dye labeling and excess dye removal steps. Deviations from key parameters such as sample incubation time and temperature, as well as pipetting accuracy during critical reagent additions can have significant impact on analytical results. Due to this complexity, it is often difficult to trouble-shoot analytical methods during their validation and transfer.
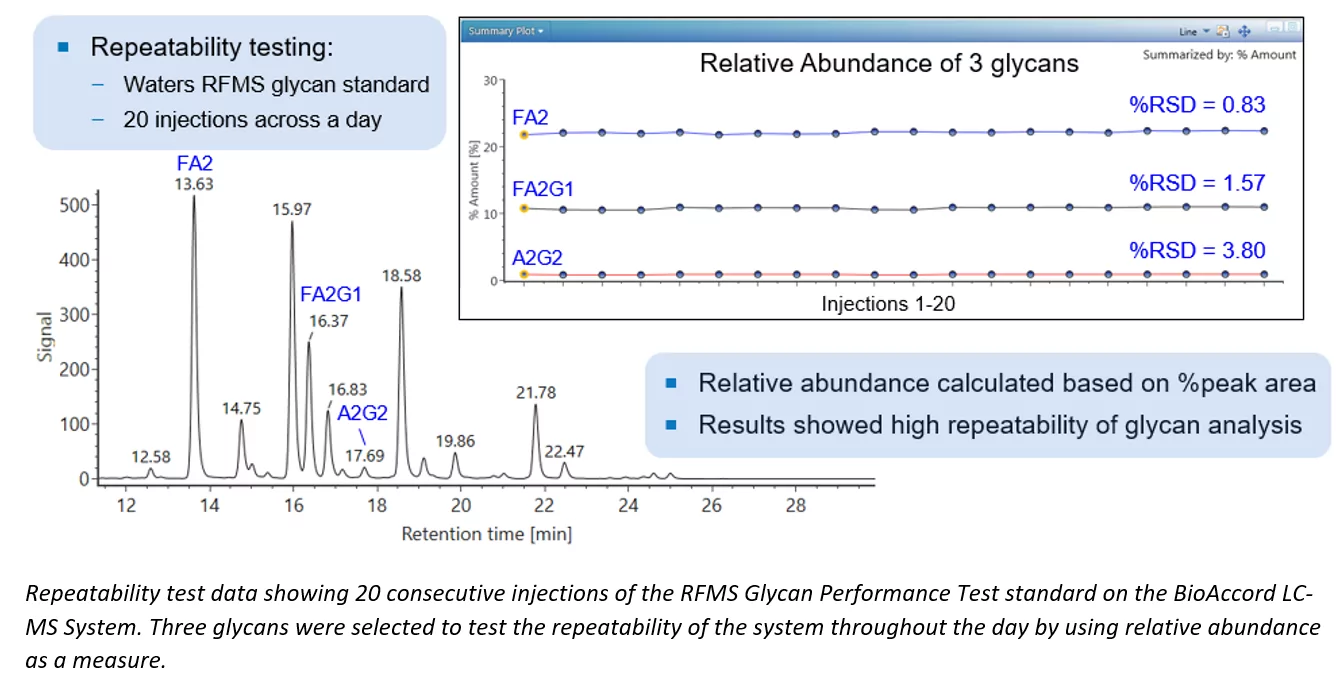
The quantification of most major glycan species is typically straightforward and accurate, with minimal variability for liquid chromatography-based methods. However, achieving consistency across different analytical runs is more challenging for minor glycan species whose retention time often shifts and relative abundance may measure below 1%. These challenges may cause inconsistency in identification and quantification, and making method transfer and reproducibility especially challenging.
Analytical protein characterization methods are used throughout a biotherapeutic’s lifecycle from the earliest discovery phases through process development and commercialization. Appropriate and effective analytical methods provide both information about the impact of process changes on the quality of pharmaceutical products and play a vital role in decision-making. Thus, the quality of analytical methods must be assured throughout their lifecycle, including development, validation, transfer, and routine use. New tools which enable these critical analytical methods are needed to ensure protocol quality and improve laboratory operations.
Laboratory personnel transferring and accepting methods can experience challenges with these complex analytical procedures, especially as they relate to biotherapeutic assays. When incorporated correctly, however, automation can be a solution to mitigate complexity and improve reproducibility within and across labs. Automation comes in many options, from assisted pipetting, to benchtop liquid handling robotics, and ultimately, total lab automation – all designed to improve workflow efficiency and reduce manual errors.
The Andrew+ pipetting robot, coupled with OneLab cloud-native software, is an innovative pairing that enables fully traceable, transferable and reproducible methods. With the Andrew+ liquid handling system, users can achieve a higher level of consistency by eliminating errors associated with manual sample preparation. Cloud based protocols enhance traceability and compliance while ensuring reproducible protocol execution across sites. The platform is also flexible in that it can be easily programmed to run up to 48 samples for N-glycan analysis without creating an entirely new method from scratch. This technology provides an easy way to automate complex workflows, allowing scientists to spend more time on higher value tasks.
Removing variability and controlling risk for all stages of pharmaceutical development is paramount across clone selection, process development, process monitoring and batch release. Bringing in the right tools – qualified kits and reagents, automated sample preparation and SmartMS – yields improved reproducibility and efficiency gains that help the industry get to critical answers faster.
Interested in automating your laboratory ?
Visit the Waters Lab Automation Solutions Page
Additional resources on Glycans and Laboratory Automation:
In the Fight against COVID-19, Science is Surging!
Setting a New Bar for Benchtop Automation
BioAccord: A Quick Look Series
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)




