Tools for Chromatographic Success: Three Ways to Support Data Quality in Routine Analysis


Avoiding the pitfalls of data quality and integrity in the Pharma QC laboratory while running routine analyses is an ongoing challenge. Regulatory violations are frequently a result of a lack of complete data, suspect data, and improper audit trails. A recent report from Deloitte stated that data integrity violations account for over 70% of the warning letters issued globally.
The correct integration of chromatographic peaks in support of data quality and integrity attracts significant inquiry during regulatory audits. One approach toward satisfying ‘right the first time’ expectations from regulators when auditing peak integration practices is modernizing and improving the performance of your chromatographic analyses. But regulatory licensing and method validation costs are increasing as lab budgets decrease, potentially discouraging you from making substantial changes to your methods.
Watch Heather Longden and Dr. Paula Hong’s webinar on demand here.>>
What simple method improvements can you make to support greater trust in the compliance of your data by reviewers, while at the same time making life easier for you?
Following are three ways to address the more common chromatographic challenges in your routine pharmaceutical analysis and advance your success.
1. Get accurate peak integration right the first time
Often, regulators find test methods inadequate in demonstrating drug purity. Why? In part because analysts struggle to get accurate peak integration right the first time. In one specific regulatory observation reported by the FDA, data was found to have been reprocessed up to 12 times, with only the final result included in the report for review by Quality Assurance.
This type of occurrence triggers concerns about whether the secured or submitted data was actually complete. It is extremely common and scientifically sound to optimize peak integration parameters; however, the cited regulatory observation was interpreted as having extensive data manipulation, especially when the manager’s response was that it was typical to play with integration parameters. There was a marked inconsistency concerning the integration of low-level impurities in chromatograms between users. Additionally, the absence of an approved protocol for manual integration led to differences in peak results. These types of test methods can lead to inconsistently integrating co-eluting peaks. Inconsistent peak integration and inadequate analytical methods can lead to a failure to detect impurities, resulting in the release of adulterated drug products to the market.
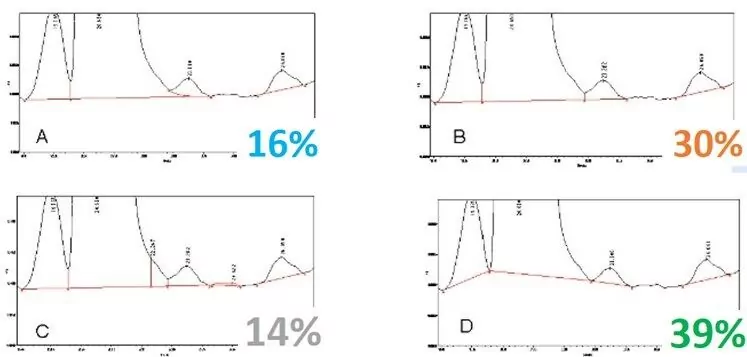
What is the right peak integration? Here are responses to a recent poll:
Most respondents selected D, which is common and easy to do, but if the peak heights change, the valley height changes, and the areas change dramatically. D underestimates the quantity of the impurity, while C overestimates. A and B are probably the most accurate measurement of the peak areas, with A more accurately integrating the impurity.
2. Understand how changes can be made to your analytical methods without requiring regulatory pre-approval
If you can improve the method so that it’s not too burdensome, you can reduce manual manipulation. This ultimately reduces any concerns regulators may have on data integrity, such as prompting suspicion that the data was being manipulated into compliance.
A common root cause of data integrity concern is that the chosen analytical methods are often old and have not been modernized since initial development. This may make them inadequate for use in a modern analytical laboratory.
Efforts by global regulators and the International Conference on Harmonization (ICH) paint a positive outlook for facilitating post-approval changes to improve analytical methods. The Product Lifecycle – ICH Q12 approach specifically includes examples as to how changes can be made to analytical methods without requiring regulatory pre-approval. In addition, global regulators and ICH are working on new guidance in Analytical Method Development – ICH Q14, which looks at incorporating more knowledge into the submission of an analytical method. What’s more, an ICH working team is revisiting Method Validation – ICH Q2 (R2) to cover enhanced validation and validation of modern analytical techniques.
Having robust methods that run reliably on high-performance systems to mitigate system suitability failure is key.
Here is what recent poll respondents had to say about system suitably test failures and method transfers:
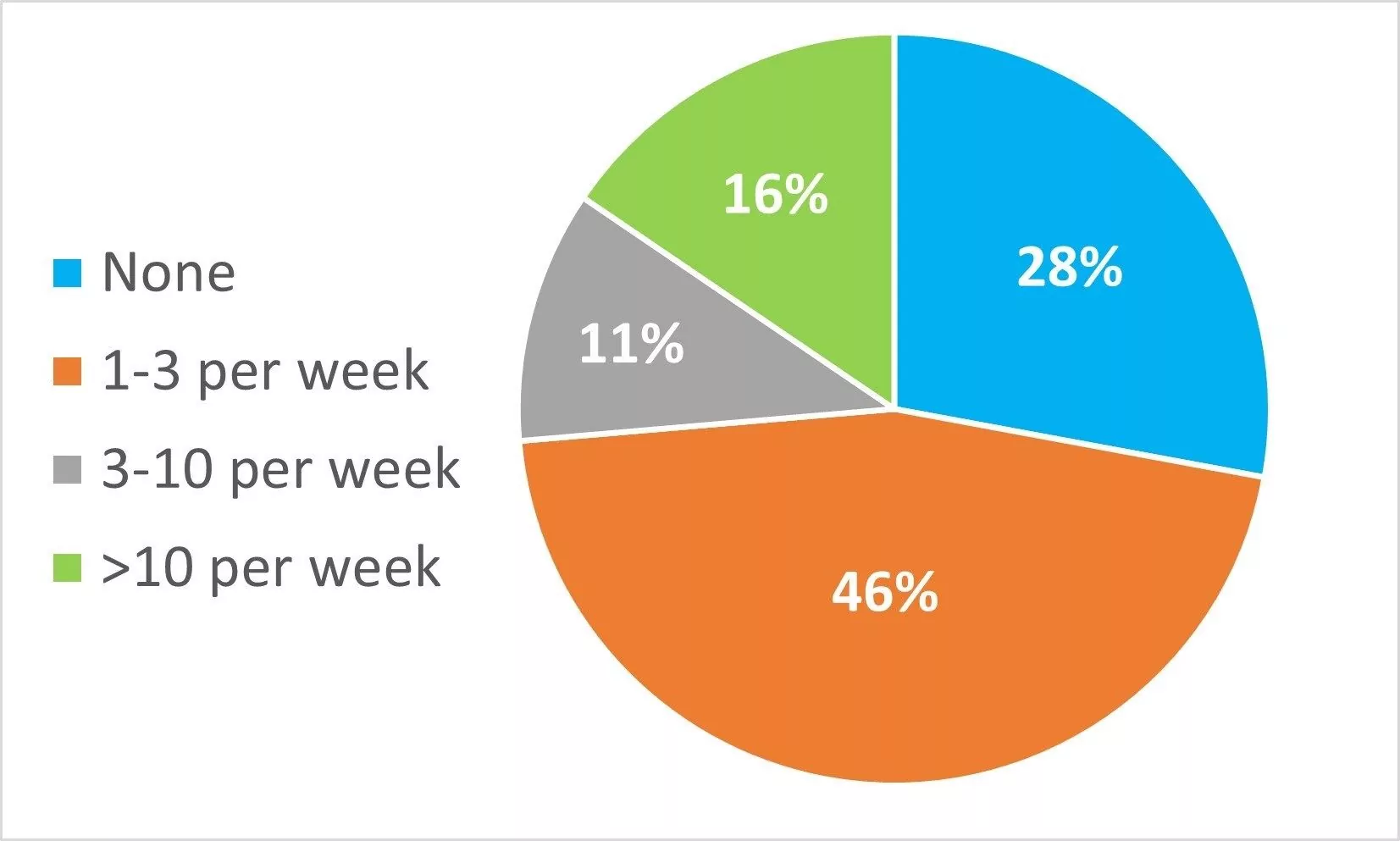
In your lab, how many sample sets per week need to be invalidated and repeated because the system suitably test fails?
Only 28% reported rarely having to invalidate and re-run results and 16% reported more than 10 sample sets per week needing to be invalidated and re-run, leading to a significant amount of wasted time and cost.
When transferring methods into QC, do you typically:
To improve method transfer success, most respondents indicated they use relative retention times as their criteria. However, there are also a significant number of laboratories that take advantage of method transfer issues to modernize their methods.
3. Make sure your HPLC system is up to the task
With its ability to improve performance, the Arc HPLC System is designed to address key chromatographic challenges. With the ability to run both existing HPLC methods and improved methods on smaller particle-sized HPLC columns, such as 3.5 µm, the system delivers highly reproducible and precise injections so you can be confident that you’ll meet your relative standard deviation (RSD) specifications without having to re-run your samples. This eliminates the need to constantly optimize peak integration parameters and reduces the time being spent investigating abnormal results.
The new Waters Arc HPLC System offers you a seamless upgrade to legacy quaternary and binary systems, featuring comparable separation quality and retention times. Upgrading to a modern system like Arc HPLC ensures you improve existing methods by providing more flexibility and boosting efficiency, with higher injection precision and backpressure limits.
Improvements in HPLC instrument performance and robustness can also contribute to the overall improvement of data quality and integrity. System suitability criteria for routine methods are readily achieved, including for USP monographs. Not only that, the Arc HPLC also implements increased backpressure tolerance to give you the flexibility to use high flow rates with smaller particle columns, reducing run time, costs, mobile phase consumption, and most importantly, achieving greater peak resolution and sensitivity. Modern instruments, such as the Arc HPLC system, are designed to provide reproducibility for HPLC shallow gradients, as typically used in many regulated (USP) methods.
The time for making improvements is now, as new regulatory opportunities for post-approval change management are imminent. The updated guidelines will influence how chromatographic methods are developed and documented, resulting in more examination and oversight of system suitability and reproducibility requirements to reduce measurement error and further ensure overall data quality.
Finding ways to easily improve your methods and ensure ‘right-first-time’ analysis gives you greater confidence in your data and confidence that regulators trust the results you are presenting or submitting. Leveraging Waters as a trusted partner helps ensure success in all areas of routine analysis.
Heather Longden, Senior Marketing Manager, Pharmaceutical Intelligence, Waters Corporation
Paula Hong, PhD, Principal Consulting Scientist, Waters Corporation
Ready to learn more?
Watch Heather and Paula’s on-demand webinar: Ensuring Data Quality and Addressing Chromatographic Challenges in Routine Pharmaceutical Analysis: Tools for Success
Plus, find more expert resources for your HPLC analysis below:
- Panel Discussion: New guidelines and modern chromatographic solutions converge to ensure data quality in the pharmaceutical laboratory: Insights from the USP and MHRA
- Article: A simple step to upgrade your routine HPLC analysis
- App Brief: Method transfer of a fast gradient from a binary HPLC to an Arc HPLC System
- App Brief: Arc HPLC System: Improved productivity and seamless transfer of an HPLC impurities method
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)