Modern Perspectives on Method Development
Hear from Dr. Fadi Alkhateeb, a Waters Senior Separations Scientist on the approach and tools used to design and develop fit-for-purpose methods.

Hear from Dr. Fadi Alkhateeb, a Waters Senior Separations Scientist on the approach and tools used to design and develop fit-for-purpose methods.

Combining BioAccord, our most capable MS detection platform, with ACQUITY Premier UPLC, our highest performance LC system, to demonstrate superior performance.

Sample handling and preparation can have a substantial effect on an analytical method’s variability and data quality. It is important to consider and address the risks associated with sample preparation as part of the method development process. This requires carefully designed and executed experiments and clear documentation in the method to give the analyst an…

In our most recent Method Lifecycle Management webinar held Improving Analytical Method Performance Through Quality Risk Management and a Design of Experiments Approach, Joseph A. Turpin, Director of Chromatography Products and Services at S-Matrix Corporation and Tran Pham, Business Development Manager at Waters Corporation, presented an overview of Quality by Design approaches to analytical method development (AQbD), the regulatory views on this approach, examples of design of experiments in AQbD, and case studies demonstrating software assisted AQbD method development.

Waters recently surveyed 100 method developers from innovator and generic pharmaceutical companies to better understand their existing challenges and views on Method Lifecycle Management (MLCM). We’ll share with you the top questions and findings from our survey results.

“Assuming continuous improvement is a worthy goal, there is every reason to improve validated chromatographic methods if you’re working with the right instrument technology,” says Eric Grumbach of Waters. See what that means for developing and transferring methods for biologic drugs.

Retaining and separating highly polar, water-soluble organic compounds has been a challenge for separations scientists who prefer reversed-phase chromatography as their technique of choice. See how Waters has addressed these analytical challenges with our T3 columns.

Chromatographers sometimes find themselves challenged as they move from analytical to preparative scales. To help gain a higher-level of understanding and knowledge of purification, check out our primer.

With access to multiple mass spectrometry systems of varying degrees of sophistication, Davy Guillarme (University of Geneva, Switzerland) wasn’t actively looking for new mass detection solutions. Nevertheless, the Waters ACQUITY QDa mass detector caught his eye.
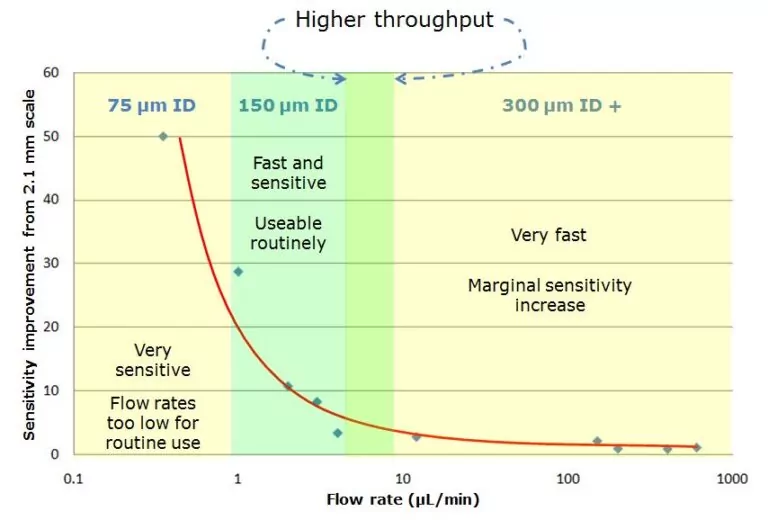
Question: When someone says microscale chromatography, what comes to mind? Increased sensitivity? Reduced sample volume? Enhanced MS sampling efficiency? Yes, yes, and yes! You might also say, “finicky,” “slow,” or “only one person in our group knows how to use it!” Maybe even, “there’s no way I can successfully transfer that method, so why…

As regulators focus in on the critical quality attributes of biotherapeutics, what can biopharmaceutical labs in late development or QC do to increase confidence in their bioseparations – other than to run more assays?

Webinar Highlights | Simplifying Methods Transfer: Novel Tools for Replicating Your Established Methods on an ACQUITY Arc System I’m Dr. Paula Hong, Principal Scientist at Waters Corporation. I recently conducted a webinar that talked about the instrument characteristics that can impact transfer of established reversed-phase methods across both HPLC and UHPLC systems. These factors include dwell…
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)