Attention Method Development Scientists! Learn about novel tools for replicating your existing methods
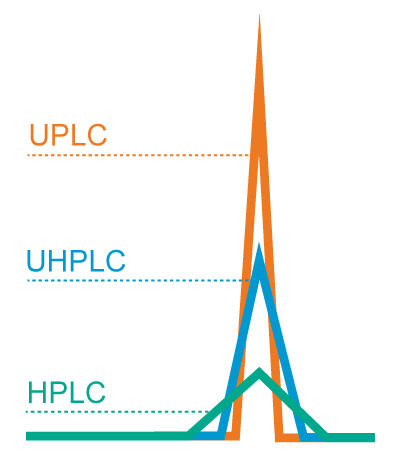
Webinar Highlights | Simplifying Methods Transfer: Novel Tools for Replicating Your Established Methods on an ACQUITY Arc System I’m Dr. Paula Hong, Principal Scientist at Waters Corporation. I recently conducted a webinar that talked about the instrument characteristics that can impact transfer of established reversed-phase methods across both HPLC and UHPLC systems. These factors include dwell…