Enhancing Analytical Productivity by Breaking Down Silos (Part 2)
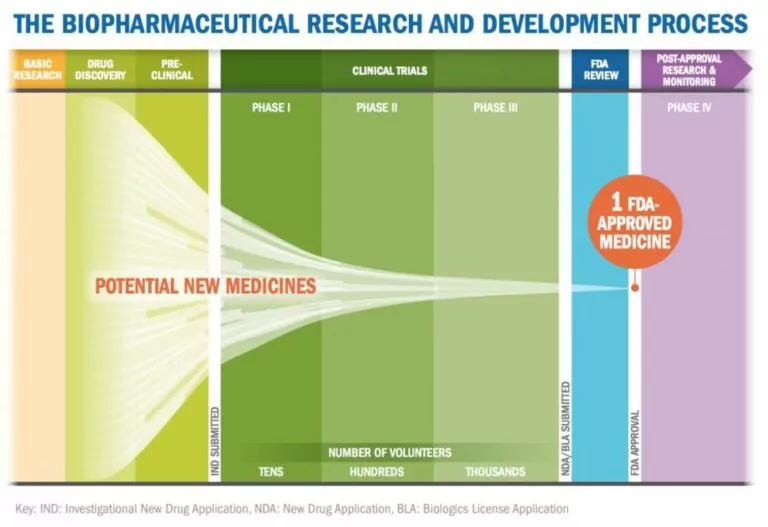
For biopharmaceutical organizations working with contract labs, it’s important to consider how data and methods will be transferred and how generated data will be managed so that it meets data integrity and compliance requirements.