Analytical Tools for Developing Biosimilars: Part 2, Peptide Analysis
Advancements in High-Resolution Analytics for Characterization of Innovator and Biosimilar Therapeutics Part 2: Peptide Analysis of Infliximab
Advancements in High-Resolution Analytics for Characterization of Innovator and Biosimilar Therapeutics Part 2: Peptide Analysis of Infliximab

Advancements in High-Resolution Analytics for the Characterization of Innovator and Biosimilar Therapeutics As the pharmaceutical industry continues to evolve its focus from small-molecule drugs to balanced product portfolios that include protein therapeutics, analytical chemists are increasingly challenged to produce routine and automated characterization workflows that move innovator and biosimilar biopharmaceutical products forward through development and…
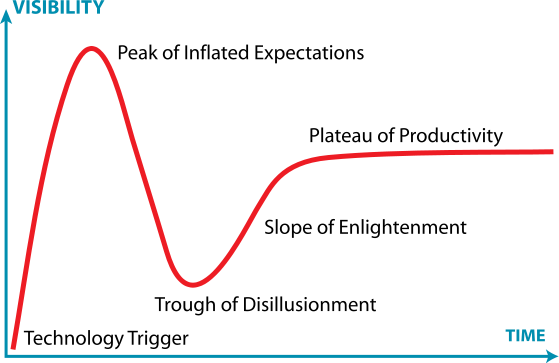
Oligonucleotide drug development has seen its share of ups and downs over the past 20 years – but why? The promise of antisense oligonucleotides (ASOs) and later small interfering oligonucleotides (siRNAs) as therapeutics that can “dial down” or even “turn off” the expression of specific genes/proteins (“gene silencing”) remains high, yet companies dedicated to their…

There is a rightfully high expectation from consumers that the amounts of compounds and ingredients that producers report in their goods are accurate down to trace levels. Whether personally or professionally, we all rely on this analytical information coming from laboratories that enables critical decisions to be made. Analytical chemists have been performing quantitative studies…
The quality issues of biopharmaceutical therapeutics are definitely different from chemical drugs because of the increased complexity of manufacturing processes and complexity of the biologic molecules themselves. There is an increasing need for detailed product characterization and control of the manufacturing process. Sheng Hou et.al. have developed and validated a practical, cost efficient, robust adalimumab…
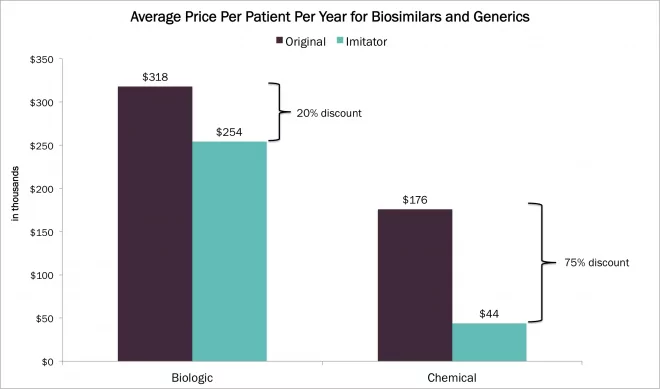
Ken Fountain, Director of Biopharmaceutical Business Development at Waters, recently returned from an insightful trip to Asia where he visited Waters customers in South Korea and attended the China Biopharma Executive Summit in Shanghai, in which Waters was a sponsor. We caught up with him and asked him to share his thoughts about the biopharmaceutical…

As regulators focus in on the critical quality attributes of biotherapeutics, what can biopharmaceutical labs in late development or QC do to increase confidence in their bioseparations – other than to run more assays?
ProteinWorks and GlycoWorks kits address more than sample prep challenges One of the most challenging parts of an LC-MS workflow happens before analytical chemists even turn to their instrument: Sample preparation. Get it right, and the analytical run performs as expected. Get it wrong, and the assay has any number of problems, from poor sensitivity…

How quickly can you go from glycosylated protein to glycan fragment confirmation? Now, analyzing released N-glycans takes about as much time as it does for Tom Brady and the New England Patriots to erase a 10-point deficit and win a football game. An exaggeration? Perhaps, but with the help of the new Waters GlycoWorks…

Close to sixty scientists from across Western Europe gathered for the Peptide Bioanalysis Forum held on-site at Ferring Pharmaceuticals A/S in Copenhagen on February 3-4, 2015. A collaborative effort between Ferring Pharmaceuticals and Waters Corporation, the proceedings kicked off with introductions by the organizers, Dr. Magnus Knutsson, Director of LC-MS/MS Bioanalysis at Ferring, and Dr….

Peptides and proteins are not small molecules. Why treat them the same? Analyzing large molecules may be one of the greatest challenges that the bioanalyst faces at the beginning of the 21st century. Employing LC-MS for the bioanalysis of large molecules requires the extraction of the target analyte from a matrix of biochemically similar peptides and proteins. However, we have…
One of the opportunities for personalized medicine is that by targeting a disease, can you eliminate the side effects of the cure? Antibody drug conjugates (ADCs) are one next-generation therapeutic approach that may have an answer. As the Cleveland Clinic notes, “Chemotherapy, the only form of treatment available for treating some cancers, destroys cancer cells…
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)