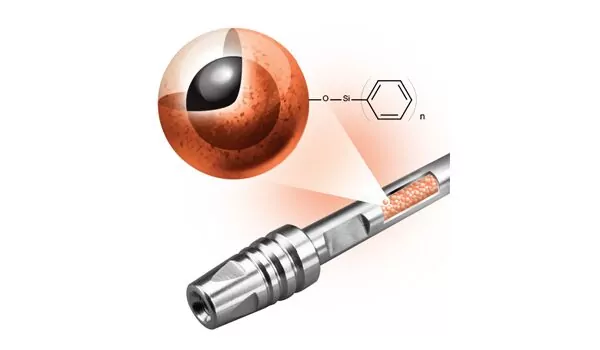
I Resolve to Get Better mAb Separations
We listened and learned how scientists separate mAbs and ADCs; then we designed a novel column for LC-MS bioseparations A critical step toward the prolific and successful use of monoclonal antibodies (mAb) as biotherapeutics occurred in 1988 when techniques were introduced to humanize these biomolecules, eliminating or reducing the deleterious patient side effects that previous…