Second Tier Newborn Screening Tests – Improving the Specificity of One of the Greatest Public Health Achievements

Newborn screening for inherited disorders has been heralded as one of the 10 greatest public health achievements of the first decade of the 21st century.1
The ability to pre-symptomatically identify newborn babies at risk of developing endocrine, hematologic, and metabolic disorders has dramatically improved or saved thousands of lives (and saved the healthcare system millions of dollars) since Dr. Robert Guthrie’s first bacterial inhibition assay was used to screen newborn for phenylketonuria (PKU). Today, the positive impact of national newborn screening programs is undisputed.
Like Dr. Guthrie’s test for PKU, the principal goal of all newborn screening tests is to rapidly and inexpensively screen the entire served population of newborn babies shortly after birth. These tests are screening tests, and must have a high degree of diagnostic sensitivity so as not to miss babies that actually do have a disorder. In pursuit of the highest possible sensitivity, some primary newborn screening tests suffer from less-than-desirable diagnostic specificity resulting in a certain amount of false positive test results (babies who are identified as being at risk for having a disorder who ultimately are found not to have the disorder). This is a conscious decision made by the newborn screening community because the harm resulting from missing a diagnosis far outweighs the potential harms associated with generating a ‘false alarm’.
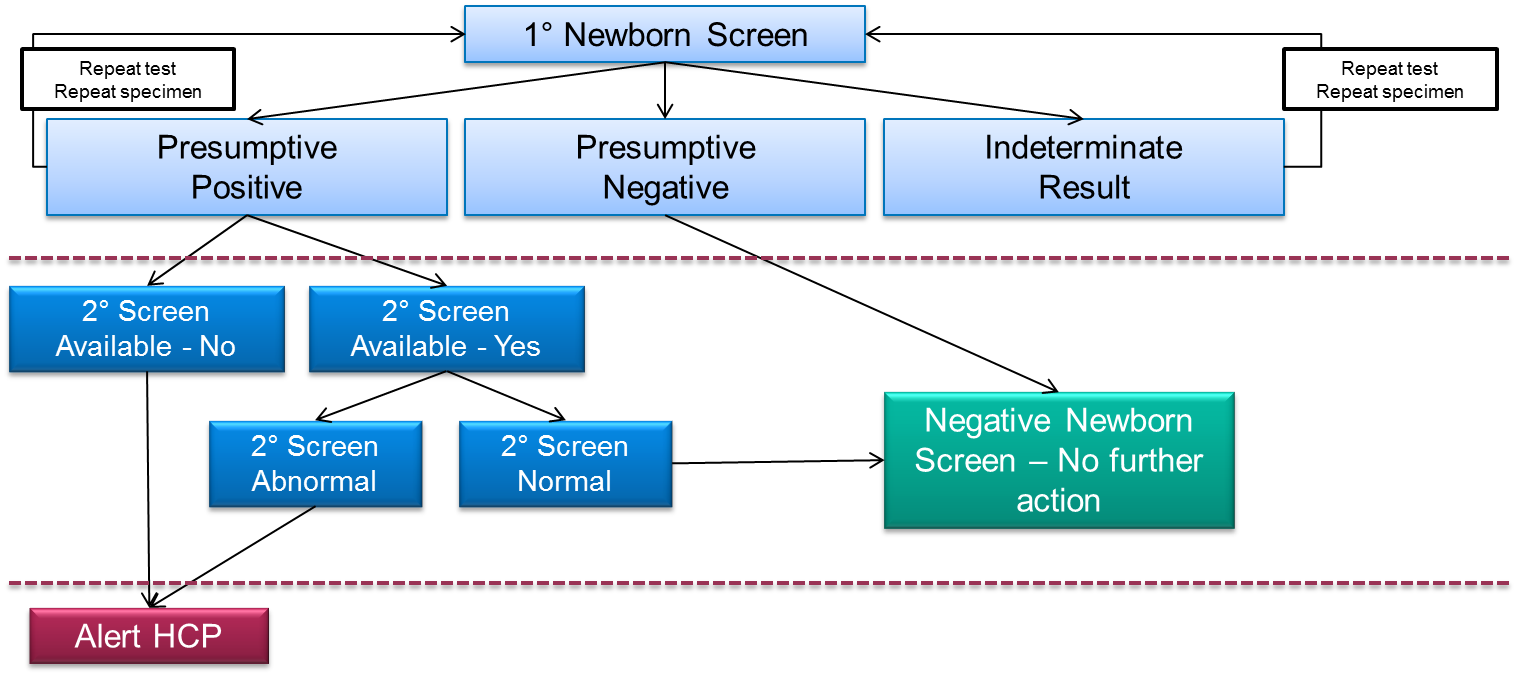
Figure 1. Example newborn screening decision tree when a second tier screening test is available. The same samples that screen positive in the primary screening test are reflexed to a second tier screening test (which is frequently based on LC-MS/MS) having a higher degree of diagnostic specificity. The goal of these tests is to provide additional information about the sample which allows the laboratory to make a more accurate judgment about the risk of disease, which should eliminate the unnecessary follow-up of unaffected babies..
Regardless, false positive test results do cause harm such as, parental anxiety2 and unneeded medical tests with their concomitant expense. Thus, the newborn screening community attempts to minimize the number of false positive test results it produces. One way of doing so is to offer second tier newborn screening tests which are applied to all positive tests that result from the primary newborn screen (Figure 1). The primary screening test could be a mass spectrometry-based flow injection analysis (FIA-MS/MS) system, but it could also be an immunoassay, or other technology platform. A second tier test is an additional test which is carried out on a new disk or disks, punched from the primary dried blood spot sample (in other words, a new sample is not taken from the baby).
Many primary screening tests have a high positive predictive value, even without using a second tier test. For these analytes, a screening result outside of the laboratory’s reference range is highly likely to indicate the target disease is present. For several others, however, a positive result from the primary screen is associated with a lesser degree of confidence. For these analytes or conditions, there is a reasonable likelihood that the positive screen will ultimately result in no disease diagnosis. Those screening tests that are candidates for the addition of a second tier test are typically those with low diagnostic or analytical specificity, either because the abnormal result is associated with several non-target disease or benign conditions, or because the primary screening platform is unable to distinguish the target analyte from various interferences.
An example of a test with an analytical interference is the detection of isobaric leucine species by FIA-MS/MS. The absence of LC separation of leucine, isoleucine and alloisoleucine, and the isobaric interference hydroxyproline, means a raised ‘leucines’ result does not always equate to the diagnosis of Maple Syrup Urine Disease (an inherited metabolic disease which is the only known cause of a raised alloisoleucine). The ideal second tier test in this scenario is a separations-based technique which resolves and quantifies all isobaric leucine species from the blood spot sample. If the alloisoleucine is undetectable and the concentrations of other branched chain amino acids are unremarkable in the second tier test, it is possible to rule-out the risk of MSUD, and there is no need to follow-up with plasma amino and urine organic acid analyses.
An example of a test result with a low positive predictive value due to low diagnostic specificity is the finding of a raised propionylcarnitine in the primary screening test for propionic or methylmalonic acidemias. Raised proprionylcarnitine can also be found in babies born to mothers with a severe vitamin B12 deficiency. When a raised proprionylcarnitine is found by dried bloodspot screening, adding on a second tier measurement of organic acids in the blood spot by LC-MS/MS3 can quickly reveal whether the metabolite pattern suggests disease in the newborn (and in this case, which one of the two disorders is more likely), or, maternal vitamin deficiency. Historically, the investigation of a raised blood spot propionylcarnitine would trigger a sequence of follow-up events involving recalling the baby for more tests, which is understandably distressing for the family, and a drain on resources for the service provider.
An example of a test for which the analytical and diagnostic specificity both contribute to a high false positive rate is the fluorescence-based immunoassay measurements of dried blood spot 17-hydroxyprogesterone (17-OHP) in the screening of congenital adrenal hyperplasia (CAH). Prematurity, low birth weight, and neonatal distress are all associated with a transient elevation of 17-OHP, as well as a host of other structurally-related steroid hormones, some of which may cross-react with immunoassay antibodies. Performing the analysis of adrenal steroids by LC-MS/MS allows the distinction of 17-OHP from other hormones not associated with CAH. It also allows multiplexed quantification of cortisol, androstenedione, and 11- and 21-deoxycortisol, which, when combined into a ratio calculation, can predict with a higher degree of certainty whether or not the raised 17-OHP is truly associated with CAH, or, if some other benign condition is present, which doesn’t need following-up.
A retrospective analysis of four years of screening data from the Mayo Clinic in Minnesota showed that introducing second tier testing for adrenal steroids following a primary immunoassay-based 17-OHP screen produced only 211 referrals, at a total cost of just over $270,000 to both the clinic and the laboratory. Had the second tier test not been in place, as many as 2712 cases would have needed follow-up, at an estimated cost of $2.3 million. Introducing the LC-MS/MS-based second tier test meant the amount on money spent on investigating unaffected babies was reduced from $8 per live birth, to only 80 cents. Given that the cost of newborn screening in Minnesota is currently estimated at $101 per case,5 this represents a huge efficiency saving. Savings which could be re-invested for the future expansion of screening.
Resources:
-
- Ten great public health achievements – United States, 2001 – 2010.
- Schmidt, J.L. et al. The impact of false-positive newborn screening results on families: a qualitative study. doi: 10.1038/gim.2011.5.Genet Med. Jan;14(1):76-80. 2012.
- Monostori, P. et al. Simultaneous determination of 3-hydroxypropionic acid, methylmalonic acid and methylcitric acid in dried blood spots: Second-tier LC-MS/MS assay for newborn screening of propionic acidemia, methylmalonic acidemias and combined remethylation disorders. PLoS ONE. Sept;12(9). https://doi.org/10.1371/journal.pone.0184897.2017.
- Matern, D. et al. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: The Mayo Clinic experience. JIMD. Aug;30(4):585-592. 2007.
- National Newborn Screening and Global Resource Center: https://genes-r-us.uthscsa.edu/resources/consumer/statemap.htm. November 3, 2014.
Read more about mass spectrometry-based newborn screening:
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)