Retained or Not Retained? How Much is Enough Retention?

In the various pieces of collateral we have published at Waters on highly polar pesticides, such as glyphosate or chlormequat, one of the main parameters we discuss is retention. But what do we mean by the word retention?
- The retention time (RT) is the time between the start of an injection to the emergence of the peak maximum of the analyte(s)1.
However, we need to know how much interaction the analyte has with the stationary phase material (the relative time it interacts with the stationary phase vs. the mobile phase).
- The retention factor (k), which was previously termed capacity factor, is the measure of time the target analyte(s) reside in the stationary phase relative to the time it resides in the mobilephase1.
If this interaction is too short, then no/little chromatography has taken place, separations will be less stable and there will be a high chance of ion suppression when LC-MS is used.
- The column void volume (v) is a measure of the internal volume inside the column packed with the stationary phase particles and can be estimated from column’s length (L) and internal diameter (ID).
- Column void volume (µl) = 0.66 x π x (column internal diameter/2)2 x L
For most of Waters columns packed with fully porous particles a ‘pore volume’ value of 0.66 can be used. Superficially porous particle (e.g. solid core), have less volume, so a value of 0.49 is often used.
e.g. for a 3.0 mm x 100 mm ACQUITY HSS T3 column, v = 0.467 mL
Knowing the column void volume and the flow rate used allows you to calculate the column void time (t0):
t0 = V/F
A more accurate answer will be found by measuring the void volume of your column when installed in the LC system by injecting a compound that you know is un-retained.
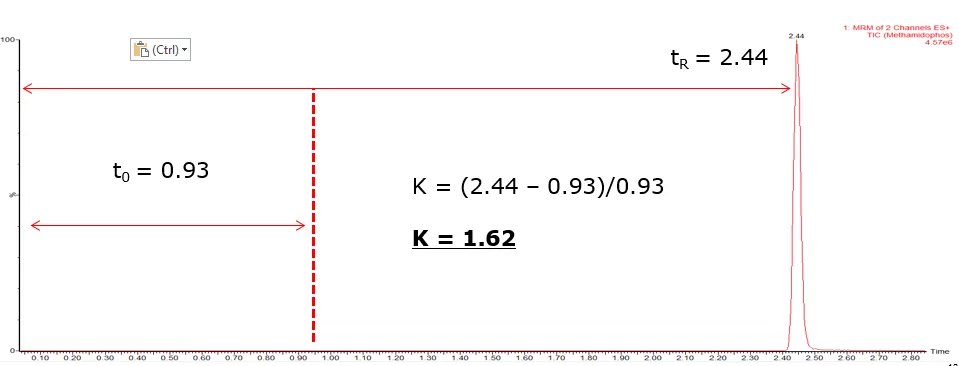
The above is an example of the retention of a relatively polar pesticide, methamidophos, using typical multi-residue reversed phase, liquid chromatography (LC) conditions.
Analytical quality control, performance and method validation guidelines such as SANTE/12682/20192 and Commission Decision 2002/657/EC3 state ‘the minimum acceptable retention time for the analyte(s) should be at least twice the retention time corresponding to the void volume of the column’.
In the above example you can see that the column and LC conditions used provide enough retention of methamidaphos, in line with the SANTE guidelines.
If we also look at the retention factor (k), the measurement of a column’s retention in relation to the column void time (t0):
k = (tR – t0)/ t0
Ensure that the earliest eluting peak has a value for k >1.0 and preferably >1.5.
When one is faced with even more polar analytes, such as ionic polar pesticides, the same rules apply, regardless of the choice of chromatographic system. It is highly unlikely that enough retention will be provided for such compounds using typical reversed-phase workflows.
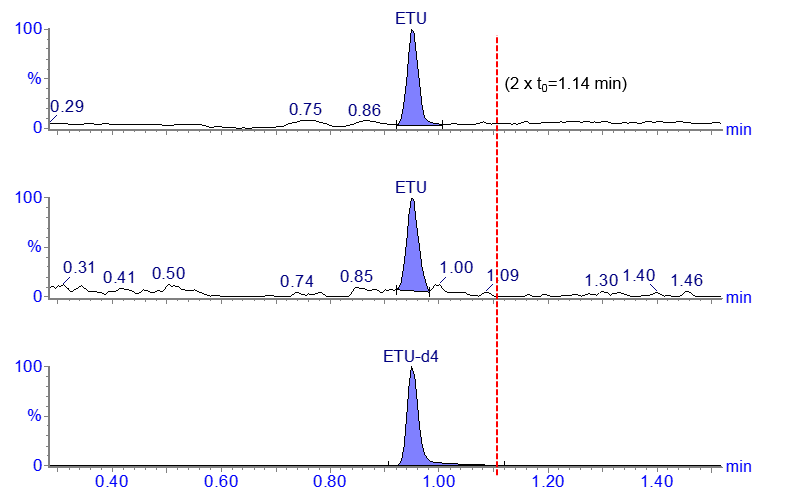
The chromatogram below shows the LC-MS/MS analysis of a highly polar compound, ethylenethiourea (ETU).
This is an early method development, example chromatogram from a HILIC column (2.1 x 50 mm, 1.7 µm) using a flow rate of 0.2 mL/min.
- Void volume for this 2.1 x 50 mm column is 0.114 mL
- Flow rate is 0.2 mL/min so t0 is 0.57 minutes (2 x t0 =1.14 min)
The retention time for ETU in this case is less than twice the column void time (t0) with the mobile phase conditions that have been used.
Options for the retention and separation of highly polar analytes can include ion chromatography, porous graphitised/graphitic carbon columns and HILIC/mixed mode chromatography. The following chromatogram shows the retention of various anionic polar pesticides using Waters’ Anionic Polar Pesticide (APP) column.
The column stationary phase consists of ethylene bridged hybrid (BEH) particles with tri-functionally bonded diethylamine (DEA) ligands. The combination of the hydrophilic surface and the anion-exchange properties of the ligands provides chromatographic characteristics well suited to the retention and separation of highly polar anionic compounds.
Under these conditions4, using a 2.1 mm x 100 mm APP column with a flow rate of 0.5 mL/min, t0 is 0.46 minutes, so the RT for AMPA, the first peak to elute, is 3.5 x t0, well within the SANTE guidelines.
So, if you either develop a LC method from scratch or implement an existing method taken from elsewhere, don’t just focus on the value of the retention time but try to ensure that retention time for the analyte is at least twice the retention time corresponding to the void volume of the column, under the conditions used.
- Appendix: HPLC Nomenclature, Waters Corporation: https://legacy-stage.waters.com/waters/en_US/Appendix%3A-HPLC-Nomenclature/nav.htm?cid=10049080&locale=en_US
- Document No. SANTE/12682/2019. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed.
- 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results and Contaminants 22(3): 245–250. http://dx.doi.org/10.1080/02652030500110618
- Determination of Anionic Polar Pesticides in High Water Foodstuffs, Waters Technology Brief 720006645 (2020)
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)