Perspectives on Pharma: Identifying and Minimizing Human Errors in Quality Control Laboratories

The one thing pharmaceutical laboratories try to avoid is atypical and out-of-specification results caused by human errors. Errors of any kind can have significant consequences for pharmaceutical companies, such as increased costs associated with remedial action, reputational damage, delays in product release, and could even impact human health.
For failures attributed to human error, finding the actual root cause is a difficult process. Root cause analysis may identify multiple contributing factors as to why the human error occurred, e.g., family and/or personal issues, equipment issues, complicated instructions or lack of knowledge. During the recent Waters-hosted roundtable discussion on the risk and cost associated with human errors in pharma quality control (QC) laboratories, we had the chance to hear perspectives from key opinion leaders in the industry, all of whom have extensive experience in identifying and minimizing human errors: Phil Nethercote, independent consultant for the pharmaceutical industry, Saji Thomas, Associate Principal Scientist, Organon LLC, and Kim HuynhBa, Managing Director, Pharmalytik.

Common types of human error
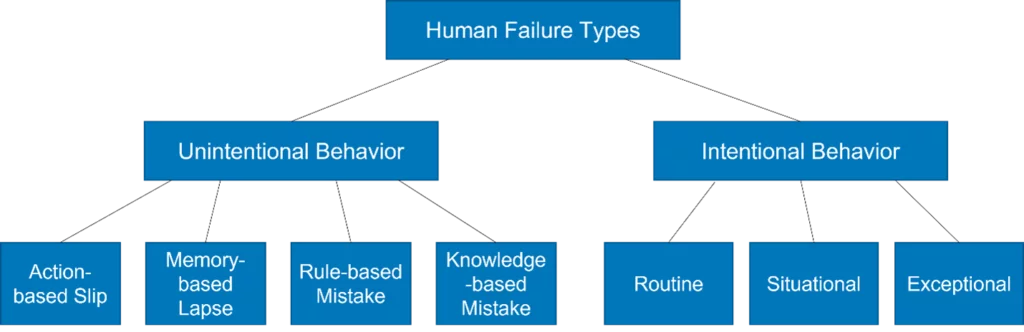
We started the roundtable discussing the root causes of human failures that occur in pharmaceutical QC laboratories. Phil Nethercote has conducted extensive investigation in this area and identified that human errors are the result of intentional or unintentional actions.
Unintentional actions can be due to the following:
- A slip while performing the task, for instance, spillage
- A memory lapse, like missing a step or inadvertently repeating a step
- Lack of knowledge, skill, or ability on how to perform the task
- Poor decision making when having to deal with a non-routine situation, for example, when the analyst fails to report that the sample is an unusual colour
Intentional actions can be categorized three ways:
- Failure to conduct the task as described
- Failure to complete the task, for example, due to lack of time
- Exceptional situations when something has gone wrong, and an attempt is made to rectify the situation rather than following proper procedure.
The picture below shows the schematic view of the example of the human failure types.
These types of human failures, unintentional or intentional, were confirmed by the other members at the round table, with a nice addition from Saji Thomas who commented that it’s important to familiarize yourself with the analytical test before you start, especially if you have not done this type of testing for a long time.
The impact of the pandemic on human errors and the associated risks
Recent and unforeseen implications of the COVID-19 pandemic introduced additional factors contributing to the number of human errors. Kim HuynhBa mentioned the increased personal stress caused by the pandemic and the impact this has had on people’s ability to concentrate on their work. She made the comment that when we’re distracted at work, we tend to rush through tasks or overlook details; we can automate some parts of workflows or methods, but not all, and we are not robots.
Saji Thomas brought to light the changes in work schedules, resulting in more shifts per day with a variety of staffing challenges such as not having the appropriate experts available when needed. With more staff away due to COVID safety protocols, less experienced team members were required to step in and conduct tasks that they may not be familiar with, increasing the potential for errors.
Minimizing human errors in QC laboratories
Once errors are identified, the compliance impact is significant, as regulators expect laboratories to conduct appropriate and thorough investigations to identify the root cause of the failure. This is an arduous process for the laboratory and might even impact the release of materials to the market. The investigational part of the failure is one aspect, but there is also the proactive part to avoid these failures in the future.

To avoid costly consequences resulting from analytical errors, these are the most important actions:
- Establish solid risk assessment procedures
- Train staff appropriately
- Ensure procedures are well-written, simple and clear
- Ensure laboratory equipment is well-maintained
Staff well-being is also a noteworthy consideration: supporting staff work-life balance, a positive mindset, and avoiding a culture of blame. Saji Thomas shared that companies with defined key performance indicators related to error reduction have decreased instances of error within a relatively short timeframe.
During the roundtable, we also heard of the importance of communication, clarity, and transparency. There are several ways that communication improvements could help reduce or avoid costly errors within the lab. For example, ensuring staff are well trained on lab procedures and are kept updated in a timely manner about changes in procedures, and, for example, any changes to labeling, signage, lab layout or organization.
We would like to thank our panelists for their participation, the lively discussion and expert insights.
Learn more about ways to identify and reduce human errors by watching our recent roundtable: Perspectives on Pharma: The cost and risk associated with human errors in pharma QC laboratories
Additional Resources:
Blog: Perspectives on Pharma: The Role of Smart Tech in Regulated Laboratories
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)


