Oligonucleotide Therapeutics on the Rise… Again
Oligonucleotide drug development has seen its share of ups and downs over the past 20 years – but why?
The promise of antisense oligonucleotides (ASOs) and later small interfering oligonucleotides (siRNAs) as therapeutics that can “dial down” or even “turn off” the expression of specific genes/proteins (“gene silencing”) remains high, yet companies dedicated to their development have come and gone, the level of investment across the industry has waxed and waned considerably, and to date only two oligo-based drugs have been approved by the FDA.
So what’s going on here?
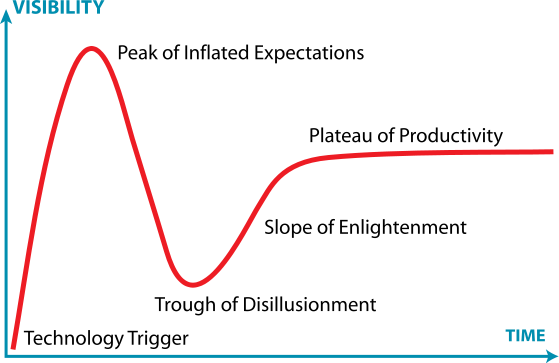
It’s a question that Dr. Muthiah Manoharan, Senior Vice President for Drug Discovery at Alnylam Pharmaceuticals, Inc., addressed head-on in his keynote talk at the Oligonucleotide Therapeutics and Delivery conference, hosted by the Cambridge Healthtech Institute (CHI) on April 4-5th in Cambridge, Mass. Dr. Manoharan provided context around the current state of oligonucleotide based drug development by referencing the “Technology Curve” (also known as the “Hype Cycle”) – a generalized development pattern that occurs with many new technologies across a range of industries – and he went on to show how the course of oligonucleotide therapeutic development fits this pattern to a T.
The “Technology Curve” or “Hype Cycle”
As shown in the accompanying image, the Technology Curve is divided into five segments beginning with the “Technology Trigger,” which spawns commercial development. For oligonucleotides, this “Technology Trigger” occurred in the mid 1990s, when the promise of antisense oligonucleotides as agents of “gene silencing” really began to take root.
“Peak of Inflated Expectations”
Next comes a wave of optimism, excitement and investment that builds up to a peak of inflated expectations. For oligonucleotide therapeutics, this peak occurred in the mid to late 2000s after many large pharma and biotech companies had invested heavily in the development of antisense oligonucleotides, and the subsequently discovered RNA interference (RNAi) technologies (siRNA, shRNA, and micro RNA), all of which effect targeted “gene silencing.”
Prominent examples of this bullishness include Merck’s acquisition of Sirna Therapeutics in 2006 for $1.1 billion, and Alnylam’s receipt of $431 million up front from Roche and Takeda in 2007 for certain rights to their RNAi technology.
“Trough of Disillusionment”
Then a sober reality sets in, and the key development challenges that need to be overcome for the technology to be commercialized, and the ever-increasing time and cost estimates to do so begin to overwhelm many. As Dr. Manoharan described, this clearly happened for oligonucleotide therapeutics in the 2009–2011 timeframe as scientists struggled mightily with the inherent instability and short half-life of these molecules, and with the equally problematic drug-delivery challenges they pose.
Early on, drug companies believed that synthetic oligonucleotides could be developed and advanced through the drug discovery funnel much faster than small molecule drugs or proteins, and could therefore quickly bolster their clinical pipelines. When this turned out not to be the case, many became disillusioned and either discontinued their oligonucleotide drug development efforts entirely, or pulled back significantly on their investments, and as a result market expectations bottomed out.
“The Slope of Enlightenment”
Today, however – as was clearly in evidence at the CHI Oligonucleotide Therapeutics and Delivery conference – the tide has turned yet again. Oligonucleotides are once again on the rise.
Dr. Manoharan conveyed his belief that while oligonucleotide therapeutics have been through a rocky and tumultuous period, they are now poised to enter the next segment of the Technology Curve: “The Slope of Enlightenment.” Thanks to a lot of great development work done by those who have forged on, many of the key challenges with stability and drug delivery have been or are being overcome.
Oligonucleotides can now be synthesized using 3rd and 4th generation chemically-modified nucleosides that vastly improve their stability, extending their half-life from two days to two weeks or more. And on the delivery side, GalNAc (N-Acetylgalactosamine) conjugation has proven to be a great way of facilitating uptake by liver cells, and Lipid NanoParticles (LNPs) are proving to be highly effective delivery vehicles for many other cell types. There’s even work being done by Avidity, Inc., and others that show lots of promise with conjugating oligonucleotides to antibodies for target delivery.
In short, the story of oligonucleotide therapeutic development is not an uncommon one. Marked by industry hype and overly-inflated expectations early on, development challenges that were once glossed over eventually led to a wave of disillusionment and contraction.
In spite of this, organizations committed to the long-term vision and promise of oligonucleotide therapeutics continued to forge on, and because of their ongoing efforts, many of those challenges have been overcome. As a result, the promise of the technology has in a sense been renewed and oligonucleotide therapeutics are now poised for a period of steady advancement and growth.
To learn about Waters’ work in analyzing oligonucelotides using LC and LC-MS, visit legacy-stage.waters.com/oligos.
Quick links to recent application notes:
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)


