Multiple Attribute Monitoring of Biopharmaceuticals Using Mass Detection


Watch the complete webinar on-demand now.
Sean started off this 45-minute webinar with an overview and benefits of this methodology and how mass detection can provide the necessary specificity and sensitivity during routine monitoring of protein PTMs and identity testing when used as a supplement to optical workflows. He spoke about the unique design and capabilities of the ACQUITY QDa Detector, as well as its ability to be deployed in compliant-ready environments that use Empower 3 Software. He also discussed how the ACQUITY QDa Detector revolutionizes monitoring of released N-Glycan profiles when used with RapiFluor-MS, a new rapid labeling technology.
Sean wrapped up the webinar by answering some of the many insightful questions that audience members submitted during the presentation. Here are a couple top questions and Sean’s responses to them.
Question: What is the difference between an accurate mass instrument like a TOF and the ACQUITY QDa?
Answer: The main differences between these two types of instruments are in their resolution and sensitivity. A TOF based instrument is primarily used for characterization purposes when identification of components, sometimes at very low levels, is necessary. The TOF is still vitally important for characterizing molecules and will remain so for the foreseeable future. In contrast the ACQUITY QDa is a quadrupole instrument, which offers unit mass resolution and lower sensitivity when compared to a TOF. For this reason, an ACQUITY QDa is not suited for characterization, but is very well suited for monitoring components which have previously been identified in characterization studies. In addition, if new components appear, the ACQUITY QDa offers the ability to assign nominal mass information about the species to aid characterization efforts. The ACQUITY QDa is well suited for routine applications where added mass information increases confidence, and improves specificity by allowing users to focus on the components of interest using familiar workflows.
Question: What is the limit of quantitation (LOQ) of the ACQUITY QDa in terms of quantity of peptide?
Answer: Unfortunately there is no single answer to this question, and it will depend on the nature of the peptide being analyzed. What is well known is that the ionization efficiency of peptides resulting from a typical monoclonal antibody digest, and other proteins as well, is that the dynamic range of response spans over at least two orders of magnitude regardless of the mass spectrometer that is used. This is a result of the properties of the peptide rather than the instrument being used. Having said that, the mass load of peptide often used in downstream optical based assays is greater than is used in characterization laboratories. This is precisely the place where the ACQUITY QDa fits. With this increased mass load we have found that the ACQUITY QDa provides the appropriate sensitivity to monitor low level peptide modifications.
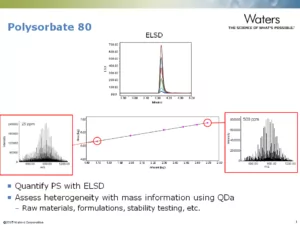
Question: Could you please comment on the PS-80 analysis? The images shown are for ELSD or ACQUITY QDa detector?
Answer: For the PS-80 analysis we used both ELSD and QDa detectors. The flow from the column is split 50/50 to flow to each detector and provide adequate sample at each concentration level tested. The ELSD data is used for quantification and was used to create the calibration curve. The chromatograms shown on the slide are those from the ELSD. The ACQUITY QDa is used to collect a mass profile of the PS-80 which are the spectra shown on the left and right of the calibration curve. These spectra represent the data at the high and low end of the calibration curve at 500 and 25 ppm respectively.
We will likely continue to hear more about multiple attribute monitoring as companies realize the benefits in productivity, efficiency, cost, and compliance that this methodology has. Waters’ ACQUITY QDa, along with RapiFluor-MS will allow organizations to collect mass data routinely for greater confidence and insight in their separations.
Can’t wait to see the complete webinar and hear other great questions from the audience? Watch on-demand now.
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)