Method Scaling in Preparative HPLC: Analytical Method &
Preparative Method
Andrew Aubin (Andy): Manager, Purification / SFx Systems Marketing Laboratory & Blogger
VOC: Voice of the Chemist
VOC: “I tried to scale up my analytical HPLC method to prep and it didn’t work.”
Andy: This is one of the most common things we hear from chemists wanting to do preparative chromatography. We also hear the following question pretty often too:
VOC: “I have a good analytical HPLC method, how do I scale it to prep?”
Andy: In this short blog, I will provide some of the basic things to do when trying to scale an analytical method to prep.
Analytical Method
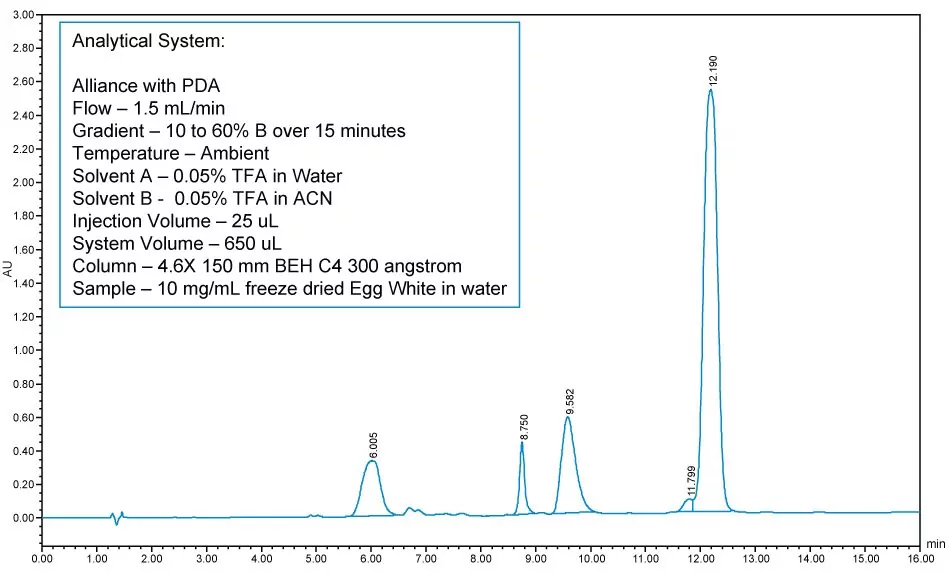
First, develop the best analytical method possible. Better analytical methods mean better preparatory methods. Mobile phases need to be the same at both the analytical and preparative scales, identical A, identical B, and have same additive concentration. Samples need to be made at the same concentration using the same diluent. Ensure that a preparative version of your analytical column is available because your column chemistry needs to be the same.
It also helps if both columns are of the same particle size and length. Loading studies can be helpful at this stage for users to confirm that adequate resolution will be maintained at the preparative scale.
Finally, keep in mind that prep systems generally do not have column heating capability, so it might be better to develop your analytical method at room temperature.
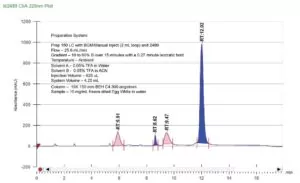
Preparative Method
Scaling your analytical method means adjusting your flow rate, gradient slope/time (including compensation for system volume), and injection volume. The easiest way to determine these new scaled conditions is to use the Prep OBD Columns Calculator.
Simply input your analytical conditions and preparative column dimensions and the calculator will generate the appropriate preparative conditions.
To learn more on scaling your method, check out these two application notes:
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)


