Lost Samples in the Container: Non-specific Binding and the Impact of Blocking Agents

My sample stayed in the solution…or did it?

What happened to my precious sample?
Such unexpected binding to the surface is called Non-Specific Binding (NSB) or Non-Specific Adsorption (NSA). These terms originally came from ligand-binding assays, where an analyte is expected to be captured only through the specific interaction between the analyte and its receptors. All other bindings are referred to as non-specific. These days the terms NSB and NSA are used to describe any binding interactions that were not planned for and the consequent analyte loss.
NSB can ruin a well-planned experiment. It can lead to inaccurate quantitative results, or even worse, you may not get any signal at all!
How to avoid this sticky problem and is it effective?
This NSB problem is not a new problem at all. It is widely known to biological scientists who have long dealt with proteins and peptides. To avoid this problem, scientists have come up with many workarounds based on these two principles:
- Principle 1 – Changing the chemical environment to eliminate or reduce the unwanted interactions.
- Changing the solution pH to reduce ionic interactions
- Adding salts to modify the ionic strength
- Adding an organic solvent to the solution
Because every analyte is chemically unique, there is no single condition that can be applied to all samples. In addition, changing the pH or adding an organic solvent may alter the properties of the biomolecule, such as its charge state or folded structure. Adding salt to the sample may promote ion suppression in MS detection.
- Principle 2 – Adding a blocking agent to cover up potential NSB sites on the surface.
- Adding polymers like PEG
- Adding detergents like Tween-20 or Triton X-100
- Adding carrier proteins like bovine serum albumin (BSA) or casein
Adding a blocking agent is basically adding impurities back into the cleaned-up samples. Detergents and large polymers are particularly detrimental to LC-MS analyses because they may alter the column selectivity and increase the background. They are known to impact the MS ionization to induce severe ion suppression. Moreover, it is extremely difficult to remove them from the column and other system components.
While it is quite effective, using a blocking agent may not be the best option for preparing LC-MS samples.
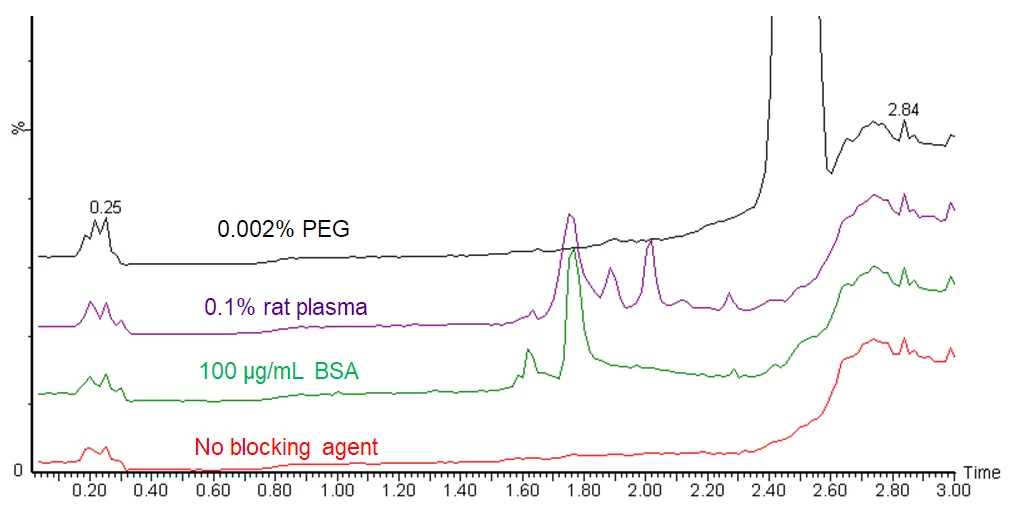
Adding a carrier protein is a less problematic alternative to using detergents and polymers. It is a more favorable solution for LC-MS samples, but is nonetheless not the perfect solution. Proteins can show up as impurity peaks in chromatograms, see figure.
One can avoid seeing impurity peaks by using a single mass monitoring or an SRM method to only monitor the target analytes, but those impurities can still impact your analysis behind the scene through ion suppression or enhancement. In full-scan analyses, they show up as extra peaks in MS spectra, complicating the interpretation. In addition, it is more difficult to handle solutions that contain a high concentration of proteins. Having both hydrophilic and hydrophobic regions, proteins in solution behave as surfactants. Protein solutions form froth, or bubbles, easily, and frothing during pipetting may lead to measurement errors.
There are some cases where a blocking agent is necessary, but it is far more desirable if you can make samples without one.
So far we thought about the problems of non-specific binding (NSB) and the pros and cons of common approaches to avoid NSB. In the next blog post, we will see how these limitation affects your sample prep workflow.
Be on the lookout for our next blog post “Sample Preparation before LC-MS Quantification of Peptides and Proteins” by Kim Haynes.
Additional resources:
Peptide and Protein Bioanalysis Boot Camp
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)