In Need of Some Reversed Phase Polar Acid Relief

Retaining polar analytes using reversed phase (RP) chromatographic conditions have long frustrated scientists. Trying to promote interactions between polar analytes and a non-polar reversed phase surfaces can often bring the most experienced separation scientist to their breaking point.
Polar acids specifically present their own set of challenges that make them particularly difficult to retain under reversed phase chromatographic conditions. Ionized silanols that are present create a negative charge on the surface of the stationary phase which repels ionized, negatively charged acids. There are some “work arounds” scientists can use to retain polar acids on a reversed phase column, but these often add another layer of complexity and potential variability to their workflows. Some of the common “workarounds” are listed here along with the problems that come with them:

Stainless steel, often associated with the analytical column and found in the system flow path, presents another issue with analyzing polar acids. Target acidic groups, ones that contain phospho, carboxylic, and/or sulfate functional groups, can bind to metal ions in the flow path. Chelating reagents are often added to the mobile phase to prevent/mitigate these analytes from interacting with the metal ions, but like ion-pairing reagents, these additives are often not mass spectrometry friendly.
Is there a better/easier solution than can be used to retain polar acidic compounds under reversed phase conditions? A solution that does not add additional steps to my workflow?
Mixed-mode or multi-modal chromatography is an answer to this question. These types of stationary phases combine multiple separation modes that can be fine-tuned to separate analytes that have different physicochemical properties. Combining reversed phase with anion exchange, provides the solution needed to be able to retain these challenging polar acids while operating under reversed phase conditions.
Over 50% of all drug molecules used in medicine exist as salts. Drugs are often formed as a weak acid or base, but this drug form is not always optimal for dissolution or absorption into your body. Without absorption, a drug cannot have a therapeutic effect, so some forms require a salt. Many medications need to be water-soluble, too. Therefore, drugs are often chemically made into their salt forms to enhance how the drug dissolves, boost its absorption into your bloodstream, and increase its effectiveness. Accurate determinations of the drugs counterions are mandatory for the release testing and quality control (QC) of all pharmaceutical salts to confirm the identity of the salt form and mass balance of the active pharmaceutical ingredient (API).
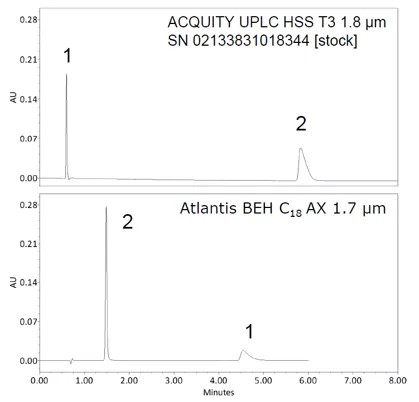
Figure 1 shows the retention and separation of the antihistamine drug Chlorpheniramine Maleate on a C18 reversed phase column that was designed to retain polar analytes, and a mixed mode RP anion-exchange column. The C18 column retains the hydrophobic basic analyte Chlorpheniramine well but cannot retain the polar acidic analyte Maleate. Maleate, a small polar acid is unretained on this column, and elutes in the void. The mixed mode RP/anion exchange column retains both analytes well. Even with a switch in elution order the Chlorpheniramine is retained via RP interactions, and the polar acid analyte Maleate is retained by anion exchange.
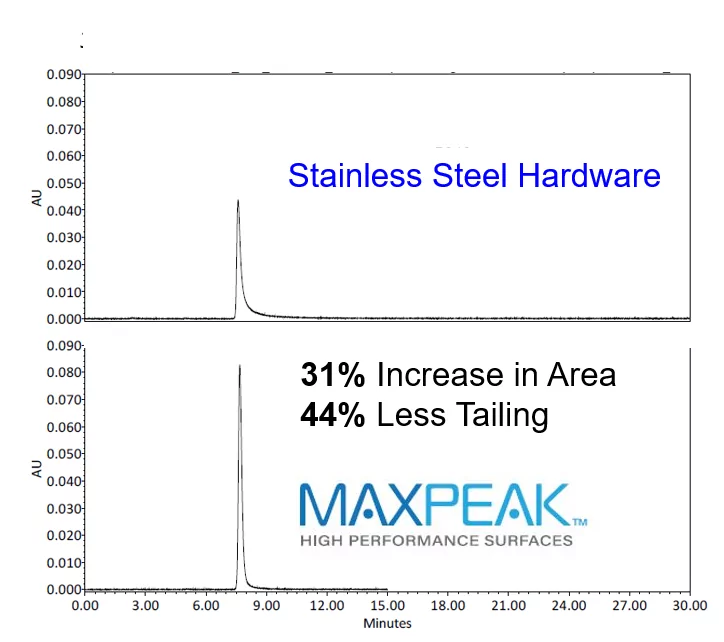
To address the acidic analytes absorptive losses to stainless steel, a new technology needed to be invented. MaxPeak High Performance Surfaces (HPS) were designed to increase analyte recovery, sensitivity, and reproducibility by minimizing analyte/surface interactions. This technology addresses problems commonly found with acidic analytes interacting with stainless steel column hardware without sacrificing the benefits of stainless steel, one benefit being the ability to operate at higher pressures.
Guanosine 5’ monophosphate is a nucleotide that is used as a monomer in RNA. The electron rich mono-phosphate functional group is attracted to the electron deficient metal oxide surface associated with stainless steel hardware. The interactions between the phosphate functional group and the metal ions causes a loss in signal intensity (area), and an increase in peak asymmetry. Utilizing column hardware that mitigates the analyte/surface interactions for these types of acidic analytes, significantly reduces the losses in area, and improves the analytes peak shape.
Challenges with retaining polar acids is only a mixed mode column away. Utilizing a reversed phase/anion exchange stationary phase in order to retain both hydrophobic and polar acids is a simple solution to what was considered a frustrating task. Columns that utilize MaxPeak HPS provides an additional benefit, increase area and improving peak shape for these acidic compounds by significantly reducing analyte/surface interactions.
For more information please check out:
https://legacy-stage.waters.com/waters/library.htm?locale=en_US&lid=135042697
https://legacy-stage.waters.com/content/dam/waters/en/app-notes/2020/720006745/720006745-en.pdf
https://legacy-stage.waters.com/waters/library.htm?locale=en_US&lid=135042507
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)



