I Resolve to Get Better mAb Separations

We listened and learned how scientists separate mAbs and ADCs; then we designed a novel column for LC-MS bioseparations
A critical step toward the prolific and successful use of monoclonal antibodies (mAb) as biotherapeutics occurred in 1988 when techniques were introduced to humanize these biomolecules, eliminating or reducing the deleterious patient side effects that previous generations of monoclonal antibodies had produced.
In light of this development, today all international regulatory agencies require a licensed mAb drug be tested for its efficacy and safety. Liquid chromatography (LC) has been and continues to be an important analytical technique to measure a biologic’s critical quality attributes due to its ability to detect and quantitate non-desired and potentially deleterious modifications to the purposely designed mAb drug. LC is also a critical analytical tool used by researchers and companies who develop biosimilar mAbs and seek regulatory approval.
Reversed-phase liquid chromatography (RPLC) is a frequently used analytical technique due to its remarkable ability to detect minor differences in the hydrophobic characteristics of biomolecules that include amino acids, peptides, and proteins. As research continues to correlate mAb structure (e.g., presence of disulfide scrambles or degree of oxidation) with biological function (e.g., half life or potency), biopharmaceutical development laboratories are under increasing pressure to obtain higher quality and more informative data from RPLC.
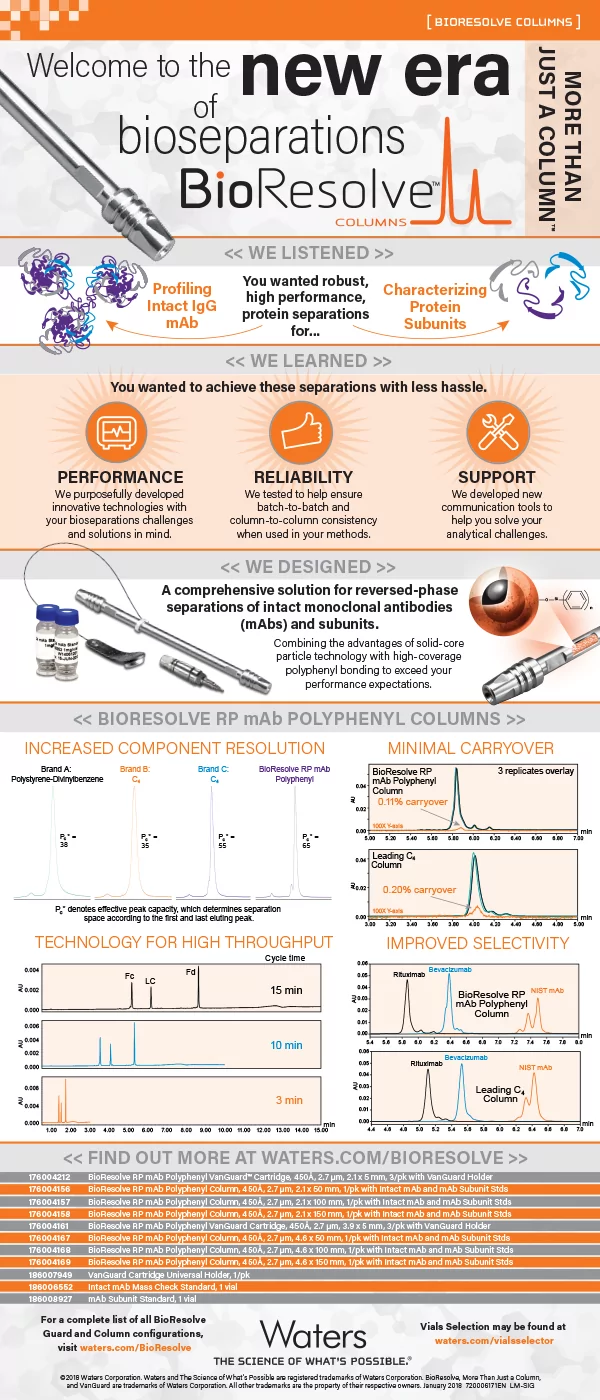
Waters has embraced this never-ending challenge to increase component resolution that is necessary to see those undesirable “needles in a haystack” by researching what laboratories want to but can’t accomplish, and then creating innovative column particle technologies, such as our latest BioResolve RP mAb Polyphenyl Columns for intact and mAb subunit analysis.
As seen in figures in the infographic below – and compared to alternative RP columns for this application space – the BioResolve RP mAb Polyphenyl Column offers robust and enhanced mAb component resolution that helps address scientists’ needs to generate high-quality data from biologics and biosimilars.
BioResolve RP mAb Polyphenyl Column: A 450Å, 2.7-µm solid core column intended for reversed-phase analysis of intact or subunits of monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) using LC-UV and LC-MS.
Jacquelynn Smith, a member of Pfizer’s Analytical Research and Development Department in St. Louis, Missouri, evaluated the BioResolve RP mAb Polyphenyl Column. She said, “The newly introduced BioResolve RP mAb Polyphenyl wider pore column provides superior recovery, separation and resolution of therapeutic monoclonal antibody (mAb) subunits and domains, as compared to other RP C4 columns on the market. This resulted in higher quality product profiles for mAbs and antibody drug conjugates, especially when hydrophobic subunits and domains were encountered. The BioResolve RP mAb Polyphenyl column addresses the key challenges of mAb and ADC subunit/domain analysis, helps toward right-first time analyses, and will be a valuable addition to our characterization toolbox.”
Learn more about the origins of our BioResolve Columns in our infographic:
See the variety of formats available for the BioResolve RP mAB Polyphenyl Column, including application-focused standards, methods, and exclusive support so you can easily and reliably achieve state-of-the-art bioseparations.
BioResolve Columns are compatibile with UPLC, UHPLC, or HPLC instrumentation from a variety of vendors, and in particular for use with Waters’ ACQUITY UPLC H-Class PLUS Bio System and ACQUITY Arc Bio System. This facilitates easy method transfer between discovery, development, and manufacturing/QC applications.
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)