BioAccord System with ACQUITY Premier: Some Things Work Better Together

It’s become clear that all elements of an analytical workflow contribute to the quality of the resulting data and the efficiency in which that data can be acquired. Beyond improvements in data quality, well-reasoned and executed laboratory workflows minimize assays failures, reduce validation costs, and facilitate efficient method transfer between laboratories. The BioAccord LC-MS System has proven to be a disruptive force for improvement in routine biotherapeutic analysis. It provides high-performance analytics to non-MS experts, along with waters_connect informatics, enabling the biopharm industry to readily deploy these integrated technologies into regulated development, manufacturing, and quality organizations.
Taking these capabilities further is necessary to realize the full vision of many companies and regulators to transform their existing analytical assays into modern platforms capable of direct measurement of biotherapeutic attributes. It is suggested by both regulators and biopharmaceutical companies that the shift to multiplexed direct attribute-based measurements will increase the quality and quantity of the product and process information that companies must have to make a safer and more consistent product.
The BioAccord LC-MS System now features the ACQUITY Premier UPLC as the default inlet, integrating the highest performance system for bioseparations into a system optimized for routine biotherapeutic attribute analysis. This new generation of attribute-based assays will likely displace a multitude of less sensitive and lower information content assays, creating savings in time and money while also reducing risks of process and quality deviations.
The Path To Unbiased Separations
The most sensitive qualitative and most accurate quantitative analyses require the efficient and accurate detection of analytes. It is long known that recovery of biomolecules from chromatographic separations can create issues in analysis sensitivity, and that non-ideal chromatographic behavior could challenge the ultimate accuracy and precision of results.
A primary contributor to these non-ideal outcomes had been the chromatography particles themselves. Silica based particles, long the base particle technology for small and large molecule separations, is prone to generating surface exposed silanol groups with a negative charge. When reversed phase separations of tryptic peptide digests are carried out under acidic conditions, the result is a set of positively charged peptides, typically with two or more charges. These peptides require stronger ion pairing agents, such as TFA, to overcome these secondary charge-based particle interactions in order to generate narrow peaks with an ideal peak shape for detection and quantification. While improvements in particle bonding and endcapping improved these separations, it was Waters’ development of a Bridged-Ethyl-Hybrid organo-silica particle technology—with intrinsically higher stability and lower silanol activity—that catalyzed the development of the modern bioseparations chemistries for biotherapeutics. The elimination of this undesired secondary interaction opened the door for building more robust and effective columns for everything from reversed phase to SEC based separations of biomolecules.
On the opposite side of the charge spectrum, biomolecules with multiple negative charges are known to interact with metal ions and metal surfaces, and in some cases produce strong interactions that lead to analyte loss and sub-optimal peak width/shape. While there are additives you can mix with the sample or spike into the mobile phase, these complicate mobile phase preparations and can interfere with optical and MS detection. The move to biocompatible systems with iron free materials such as titanium and MP35N, can provide salt/pH protection from aqueous based separations modes and surfaces with significantly reduced metal ion surface exposure.
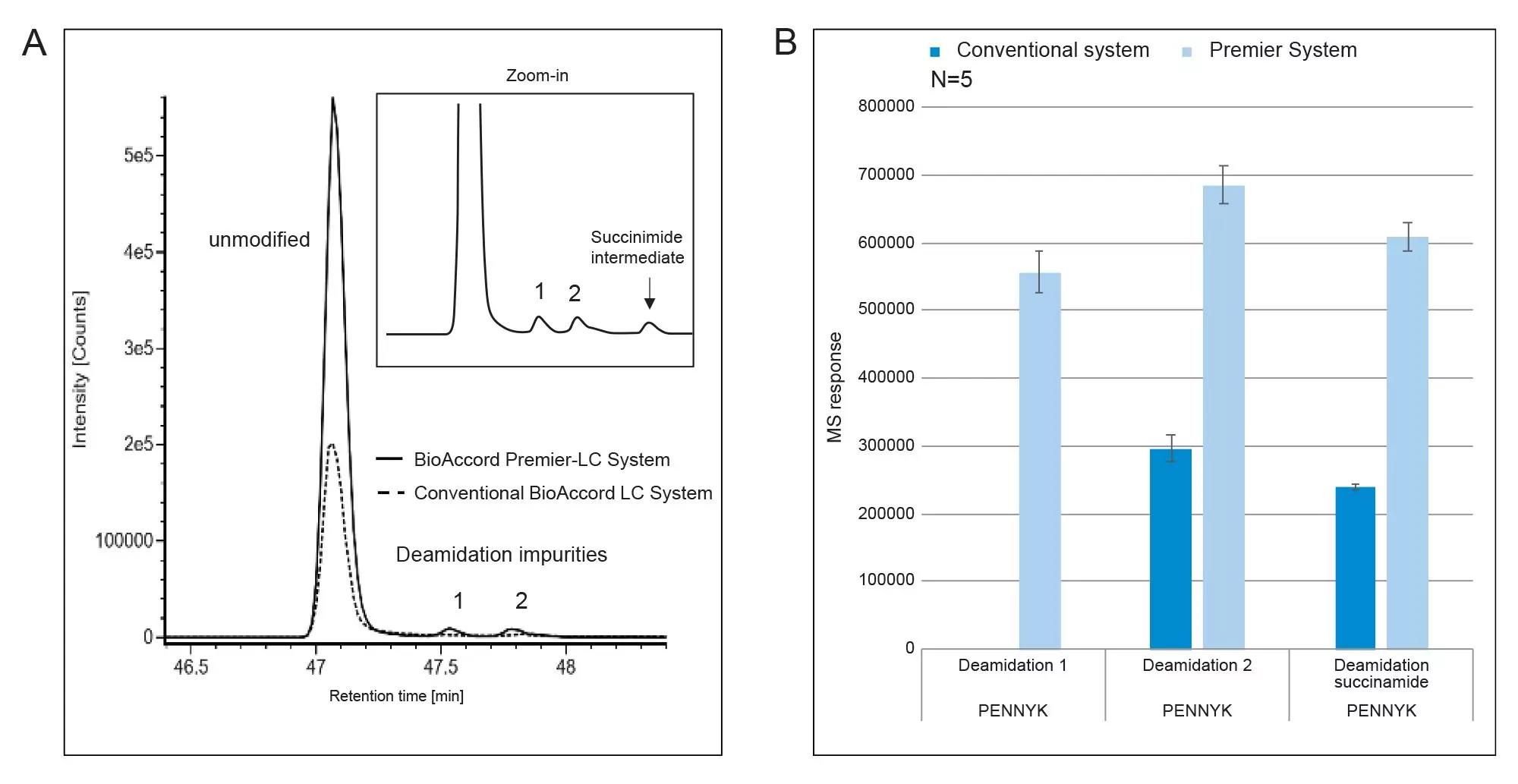
Waters used these biocompatible systems as the foundation for the next generation of UPLC bioseparations platforms, ACQUITY Premier. These systems make use of MaxPeak High Performance Surfaces (HPS) Technology. This technology further modifies the biocompatible surfaces of high surface area components and therefore protects metal sensitive analytes from interaction with metal surfaces—without degrading the underlying separation processes. These systems, when paired with HPS, protect Premier Column chemistries containing hybrid particle-based chemistries. This creates an optimized platform for the most sensitive, robust, and reproducible biotherapeutic analysis workflows on the BioAccord System.
Realizing The Benefits Of ACQUITY Premier On The BioAccord System
With the introduction of the ACQUITY Premier System, several application notes were generated demonstrating the exceptional performance of Premier Systems for both aqueous and organic solvent based optical bioseparations, and as an MS-inlet. Additional applications have been generated documenting the differentiated performance of just the Premier Columns for several bioseparations modes.
As part of verifying the integrated BioAccord System with ACQUITY Premier, Waters confirmed the methods used for application-focused installation testing and applications training, and documented specific common analyses where the integrated system and Premier chemistries demonstrate superior performance:
- Analysis of Oligonucleotide Impurities on the BioAccord System with ACQUITY Premier
- Enhanced Recovery of Released Glycans Using QuanRecovery Sample Vials with MaxPeak High Performance Surfaces (HPS) Technology
- The BioAccord System With ACQUITY Premier for Improved Peptide CQA Monitoring
- Released Glycan Analysis of Erythropoietin Using the ACQUITY Premier Glycan BEH C18 AX Column and BioAccord System with ACQUITY Premier
- Improving Released N-glycan Analysis in Biotherapeutic Development Using the ACQUITY Premier Solution with MaxPeak High Performance Surfaces (HPS) Technology
The ability to realize greater response from the system for these classes of molecules, and the robustness and producibility gains from consistent recovery and chromatography of all the components in these complex bioseparations, support the adoption of this integrated LC-MS system platform.
A Commitment To Controlled Innovation
Innovation is both necessary and inevitable to deal with the growing complexity of traditional biotherapeutic modalities and the new challenges arising from nascent cell and gene mediated therapies. Waters continues to invest in both basic and applied R&D from automating sample preparation to producing new data analysis workflows to meet these evolving challenges.
The BioAccord System was intended to supplement the capabilities of development groups for product and process characterization and was purposely built for attribute based biotherapeutic analysis in late development, manufacturing, and quality organizations. These latter regulated environments demand qualified instruments and validated methods that require stable platforms over extended periods to avoid unnecessary disruption and expense. This is an ethic that Waters has long embraced from decades of support for chemistry products, to the careful release and updating of laboratory and enterprise class informatics products such as Empower, NuGenesis, and waters_connect. 
It’s called the BioAccord System with ACQUITY Premier. Learn more.
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)