Binary vs Quaternary Pumps: How is a Gradient Created?
How is a Gradient Created?
Any liquid chromatograph (LC) is an amalgamation of a certain set of components that delivers to the scientist a monograph of the sample they are analyzing. Traditionally, an LC system consists of a pump, an injector, a column heater, a detector, and even more commonly these days a mass spectrometer (MS) or mass detector. In my last blog post, I discussed the differences between common optical and mass detectors and their uses.
I would like to talk about pumps and some of the design nuances that can affect how you run your LC.
A Quick History Lesson
Today’s LC methods come in two different flavors: isocratic or gradient. Originally, all LC methods started off using isocratic pumps, because the first detectors (refractive index, or RI) could only be used with a single solvent. But with the advent of the UV detector, separation methods moved towards gradient analyses to improve peak shape as well as to decrease the analysis time for samples. Thus, when a scientist is developing a modern gradient LC method, one of two styles of pumps will be utilized for the analysis: binary or quaternary.
Before we dive into the explanation of each type of pump, here are some fun facts. The word isocratic originates from the following Greek origin:

The Latin breakdown for binary and quaternary are as follows:
Principals of a Binary Pump
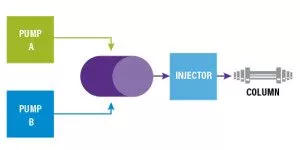
In a binary pump, we can only pool two solvents together at a time to create a gradient. This is accomplished by having two independent pumps, with each pump providing flow for a specific solvent. The solvents are then combined in a mixing chamber that is located after the pumps. This creates a high-pressure proportioning environment because the solvents are already under pressure before they reach the mix-point where the mixing occurs.
As an example, let’s say we have a method with that runs Solvent A and Solvent B at a ratio of 70:30. If our flow rate is 1.0 mL/min, the pumps would deliver 0.70 mL/min for Solvent A and 0.30 mL/min for Solvent B. (Figure 1)

Principals of a Quaternary Pump
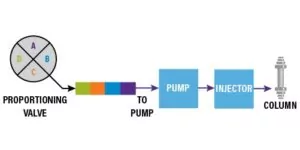
A quaternary pump has one pump, which is used to deliver the mobile phase to the system. The gradient is created through a device called a proportioning valve. The proportioning valve delivers the gradient by opening a “valve” (I bet this reveal floored you) and delivers a “packet” of solvent to the pump head. Once the packets of solvents are delivered to the pump, the plunger draws the solvent into the pump head(s) and creates a turbulent environment where the mobile phase mixes together.
Because the mobile phase is not under pressure at the point where the proportioning valve introduces the packets of solvent, quaternary pumps are typically considered low-pressure proportioning. Some systems will add a mixing chamber post pump, to enhance further homogenization of the mobile phase before delivering the gradient mixture to the column.
As an example, let’s say a scientist wishes to run a method that runs Solvent A, B, C and D at a ratio of 25:25:25:25. The proportioning valve would open the valve of each solvent to deliver an equivalent sized packet of solvent to the pump heads. (Figure 2)
Conclusion
Gradients can be created and delivered in different ways. The initial obvious observation is that the quaternary pumping system provides flexibility by allowing a scientist to utilize and mix up to four (4) different solvents at the same time. On the other hand, a binary system is restricted to only two (2) different solvents at the same time. However, there is more to this than just that simple assessment.
Waters technologies:
- Binary chromatography systems, including HPLC, UPLC and microflow LC
- Quaternary chromatography systems, including HPLC, UHPLC, and UPLC
- Isocratic solvent management for polymers
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)


