

In July 2018, the European Medicines Agency (EMA) reported the recall of a number of products containing the active pharmaceutical ingredient (API) valsartan due to contamination with a known carcinogen, dimethyl nitrosamine, also referred to as N-nitroso dimethylamine (NDMA). This action set off a chain of events that led to similar notifications of recalls for…
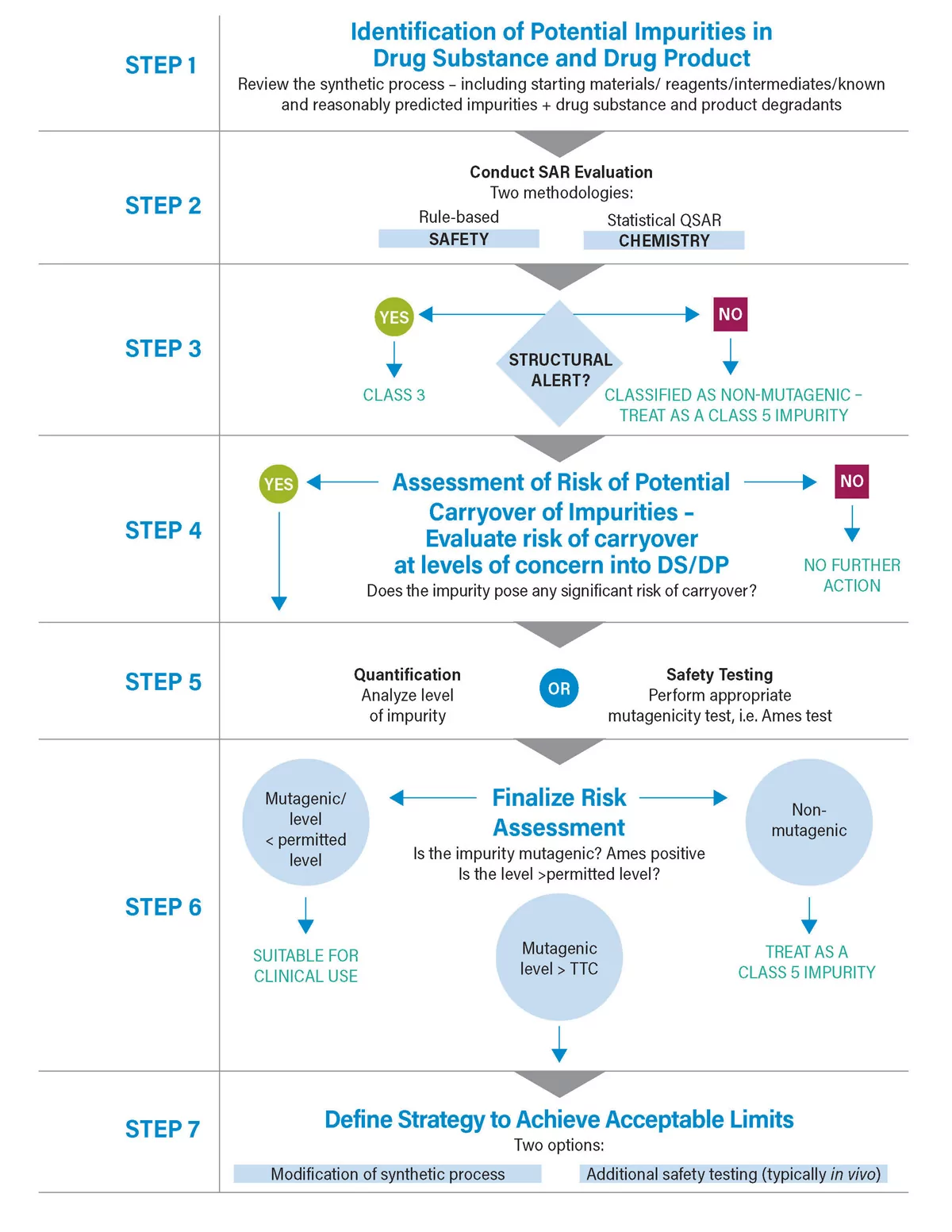
Since the early 2000s and the advent of the first guidance relating to mutagenic impurities developed by the European Medicines Agency (EMA), it has been necessary to assess the risk posed by mutagenic impurities. Although initially it was thought that avoidance was an option, it quickly became apparent that this was not a viable strategy. …