Accelerate Complex Materials Formulation Screening with Mass Spectrometry Analysis

The analysis and monitoring of active ingredient/additive chemical profiles plays an important role in materials formulation screening across industries like automotive, polymers, fast-moving consumer goods (FMCG), oil and gas, and fine and specialty chemicals. Determining relative levels of active ingredients and specialty chemicals, tracking breakdown products and degradants, and measuring potential impurities provides the key to gaining insights into advanced formulations performance during testing regimes.
Advanced formulation designers currently rely on analytical techniques such as Fourier Transform Infrared Spectroscopy (FTIR) and various spectroscopic methods to measure the chemical behavior of formulations, but these techniques do not provide comprehensive insight into specific chemical changes around active ingredients and additive chemical profiles. A well-established technique that provides specificity when measuring chemical entities is atmospheric pressure mass spectrometry, which helps identify the chemical formula and component structure in complex mixtures like advanced polymeric formulations and measure the difference in component amounts between samples.
However, operational complexity and relatively high pricing of MS instrumentation have posed barriers to adoption in the chemical manufacturing industry for materials formulation screening. RADIAN ASAP is a novel, dedicated direct analysis system that addresses the mainstream market’s need for rapid, easy, low-cost analysis of complex liquid and solid samples. The RADIAN ASAP system and its informatics solutions can streamline three universal workflows for the chemical manufacturing industry:
- Advanced materials R&D
- Chemical manufacturing process analysis
- Raw material authenticity/release testing
RADIAN ASAP in Advanced Materials R&D
With RADIAN ASAP, you can apply a thermal gradient temperature ramp that separates common mixtures of bulk polymers, processing aids, additives, and other active ingredients based on their different boiling points. In figures 1 and 2, we easily separated and identified both the polymeric material and the processing additives of a polymeric formulation using this approach. Analytical scientists or formulation engineers can use this quick, simple technique to easily observe the presence of these components and any changes they undergo, simplifying the formulation design decision-making process.
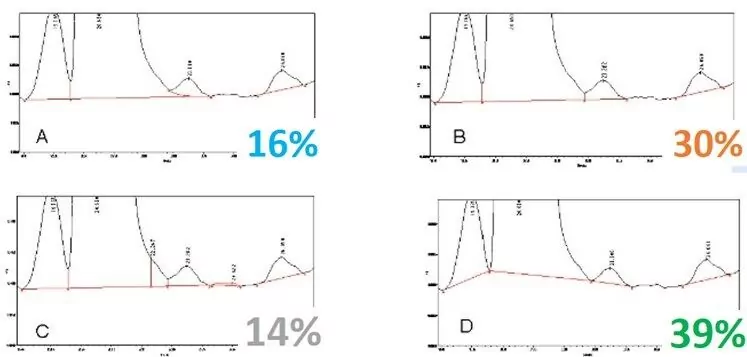

RADIAN ASAP in Chemical Manufacturing Process Analysis
Monitoring industrial batch chemical manufacturing processes and plant equipment in real-time can help fine and specialty chemists and chemical engineers:
- Design and control reactions in process vessels, increasing product yields and purity and reducing side reactions and by-products
- Reduce costs and risks associated with unexpected processes
- Increase laboratory and plant efficiency with reduced decision-making time due to rapid turnaround in sample analysis for reaction monitoring
In figures 3 and 4, a manufacturing process for a substituted amino propanol is analyzed using the RADIAN ASAP system.


RADIAN ASAP in Raw Material Authenticity / Release Testing
Quality control testing is crucial to the production of valuable advanced formulations. With RADIAN ASAP and its advanced informatics solutions, you can verify critical parameters of raw materials and ingredients — a fundamental part of this process. One of those advanced informatics solutions, LiveID™, uses an optional additional informatics tool available for RADIAN ASAP, uses statistical models to classify samples so quality control scientists can investigate authenticity, adulteration, and the quality of ingredients or raw materials. Authentic or verified samples can be used to create and validate a statistical model, and statistical models can test unknown or verified samples for live classifications, improving efficiencies with real-time library matching workflows.
Learn more about RADIAN ASAP
Additional Resources:
The Expanding Role of Mass Spectrometry in Biotherapeutics
Supercharging Mass Spectrometry in the Fight Against SARS-COV-2
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)


