A View of the Biopharmaceutical Industry in Asia — an Interview with Ken Fountain
Ken Fountain, Director of Biopharmaceutical Business Development at Waters, recently returned from an insightful trip to Asia where he visited Waters customers in South Korea and attended the China Biopharma Executive Summit in Shanghai, in which Waters was a sponsor. We caught up with him and asked him to share his thoughts about the biopharmaceutical industry in Asia.
Q: Based on conversations and observations during your trip, how strong is the biopharmaceutical industry in Asia?
A: Asia is a large growth engine for both the pharmaceuticals and biopharmaceuticals industries. In China and South Korea specifically, the focus is dominated by the development of biosimilars. Already, several biosimilar drugs have been manufactured and put on the market in these countries. The region is putting a lot of effort into advancing biosimilars and other biologics.
In fact, it’s happening at such a rapid pace that some estimates put future sales of these biosimilars greater than those of small molecule generics. Certain drugs, such as Antibody Drug Conjugates (ADCs), are now being targeted for biosimilars even though their patents won’t expire for years.
We’re also seeing many global pharma companies partner with academic institutions and local biopharmaceutical companies in this region. They are establishing innovation centers and manufacturing facilities in order to accelerate biosimilar development and approval which is also contributing to Asia’s rapid growth in the industry.
Q: What else have you learned about the region’s efforts to advance biosimilars and other biologics?
A: Probably the biggest discussion is around regulations. What will be the regulatory guidelines that define “similarity” for a biosimilar, both within the countries as well as out in the global market? The outcome of these discussions will determine the regulatory path for biosimilars produced and marketed for the local population versus ones produced for sale in Europe and the Americas.
In China, regulations around biologics are changing very rapidly to be in line with global harmonization standards. In addition to the work on biosimilars, I mentioned that there are a number of global pharma and local companies partnering to develop new drugs. To that end, the Chinese FDA is establishing an accelerated path for review. Furthermore, the Chinese government is investing heavily into early discovery efforts. Primary efforts are focused on better medicine for the Chinese population.
The biopharmaceutical industry is fairly well established in South Korea, with the world’s first antibody biosimilar Remsima, produced by Celltrion, approved there in 2013 (see related Waters application note). There’s strong partnership between private industry and the government with central testing labs that provide characterization services as well as validation services for new technologies, which can be adopted into regulations.
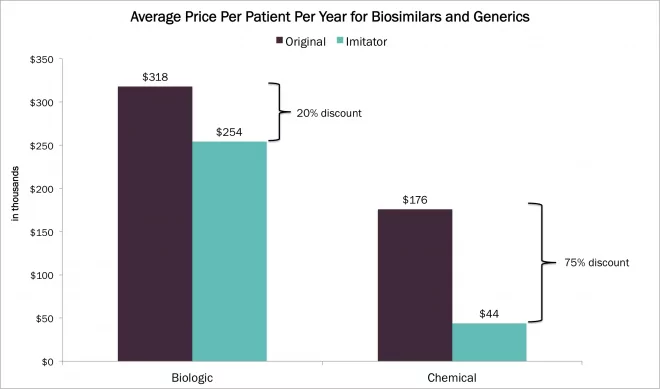
Recently, Korea’s Samsung Biologics revealed its goal to become the largest manufacturer of biologic drugs in the world by 2020. There have been many articles on Samsung’s latest investment in biopharmaceuticals, recently capped by Samsung Bioepis’ approval of their Enbrel biosimilar in both South Korea and Europe (see related Waters application note). These efforts could have significant implications for the industry, specifically around the global pricing structure of biosimilar medicines, which are currently sold for approximately 25% less than the reference products.
Q: What are some of the exciting innovations and technology advancements that you saw in Asia?
A: Due to the need for full characterization of biologics to prove similarity to the reference products (see related Waters webinar), both South Korea and China are investing in many of the same technologies used in western pharma companies—namely, liquid chromatography, mass spectrometry, and capillary electrophoresis. This includes high-resolution mass spectrometry for doing in-depth chemical and structural characterization, which are key parameters for determining similarity of what they are producing versus the reference products.
As the biopharmaceutical industry looks closely at innovations in manufacturing and facility design, Asia is one of the key places where these new facility designs are tested and implemented.
With regards to production, biopharmaceutical giant Amgen opened a single-use manufacturing plant in Singapore in 2014. These plants use less energy and water in comparison to conventional plants, but produce the same output. They are smaller, more flexible, and save in operating costs.
Q: Are Asian markets going to influence biopharmaceutical development around the world?
A: I think the Asian market has already proven their importance to the industry. As I mentioned, several biologic drugs—many of the biosimilars on the market today—have been developed and commercialized in Asia, sometimes at record pace. That development of biosimilars is accelerating, and Asia plays a critical role in driving discussions around pricing and regulatory guidance.
In the future, Asia will be a key player as an engine for development of new drugs, the technologies needed to characterize them, and advancements in biologics production and release testing.
Q: What is Waters doing in the area of biosimilars?
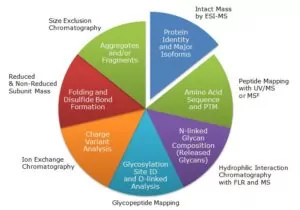
A: Waters has been involved in applying our biotherapeutic characterization capabilities to the area of biosimilars for some time, specifically as it relates to determining similarity.
Currently, we are working with regulatory agencies and biosimilar drug developers and manufacturers to understand the next set of challenges they face, and how Waters technology can address those challenges to help get these drugs to market faster. This includes education on what biosimilars are, how similarity is defined, and what LC-MS can do to help accelerate biosimilar development and commercialization.
In 2016, we will be releasing applications for impurities (e.g., host cell proteins) and higher order structure analysis on monoclonal antibody (mAb) biosimilars, as well as using LC-MS based solutions for similarity assessment of non-mAb biosimilar drugs such as insulin and erythropoietin (EPO).
For more information on Waters technologies and tools for biosimilar analysis, please visit legacy-stage.waters.com/biosimilars.
Follow Ken on Twitter: @mAb_dude
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)


