5 Insights: Protein Quantification Using the Surrogate Peptide Method

Bioanalysis of Biologics
What can you get all the top Pharma and CRO’s scientists to agree on? That the biopharma industry is growing and protein quantification for bioanalysis is difficult – but you have options. The following insights will bring you up to speed on current challenges, issues, and solutions.
1. Biopharma is Growing Fast
Biopharmaceutical drug development is experiencing rapid growth. More than 600 biologics have U.S. FDA approval and over 50 monoclonal antibodies (mAbs) are approved in the US and EU [1], with at least another 300 mAbs under development [2]. This growth forces scientists to tackle a variety of challenges not present in small molecule quantification (like these).
2. Current Bioanalytical Approaches Have Limitations
Protein quantification involves complex and time-intensive workflows and arduous method development. Ligand binding assays (LBAs) have traditionally been employed for quantification because of their high sensitivity, low cost, and high throughput. Despite these advantages, LBAs have limited linear dynamic range, carry a risk of cross reactivity across metabolites/analogs, and maintain difficulty with multiplexing. Also, if a new LBA antibody is required for your protein of interest, the development time and cost is significant.
3. LC-MS Offers Advantages
Chromatography and mass spectrometry (LC-MS) have emerged as a highly effective approach for protein bioanalysis, particularly for drug discovery, early development, and pre-clinical studies. LC-MS has a number of advantages compared to LBAs, including improved specificity and accuracy, multiplexing (can detect multiple proteins in one run), broader linear dynamic range, faster method development (often days to weeks), and no need to develop protein-specific antibodies.
LC-MS quantification of proteins offers a variety of options, each with advantages and caveats. Using mAbs as an example, there are two main workflows in the tool box: Intact and digested analyses. The advantages of each depend on each scientists’ particular requirements and restrictions. While this blog is focused on the surrogate peptide approach, it is worth mentioning that intact mAb analysis is possible (even at the 1 ng level).
For intact analysis via LC-MS, a high resolution mass spectrometer (HRMS) like a QTof MS is ideal because it offers the widest mass range (>150 Kd) and very high resolution. Intact mAbs can be challenging to ionize because they have highly charged envelopes, they attract salt ions, and are highly glycosylated, which requires extensive sample prep.
HRMS instruments also provide the opportunity to perform qualitative analyses and to use ion mobility as an additional separation stage. For more information about the benefits of ion mobility, see this recent blog post by Dr. Nigel Ewing.
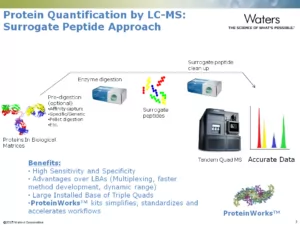
4. The Surrogate Peptide Approach
The surrogate peptide approach has become the most commonly used strategy for MS-based protein quantification due to its high sensitivity and specificity, and the wide availability of triple quadrupole (TQ) mass spectrometers in bioanalytical laboratories [3].
In this approach, the target protein is digested into smaller peptides. Then one or more unique peptides with good selectivity and sensitivity are used as a proxy (or surrogate) measure of the target protein. The surrogate peptides are then analyzed by LC-MS.
Digestion is essential to leveraging the advantages of TQ mass spec, as the mass-to-charge ratio is 2048 m/z and total mass range is approximately 6000 Da. An intact mAb has a very large mass, approximately 150 kD, much too large for an intact analysis on a TQ MS.
Finally, due to their prolific use and high sensitivity, TQ mass spectrometers are viewed by global regulatory agencies as the gold standard for quantification and are thus ideal for the surrogate peptide method.
5. Complex Sample Preparation Isn’t a Barrier
Whether using LBA, intact, or digestion, protein quantification workflows, including sample preparation requirements, are inherently time-consuming. The surrogate peptide approach is no exception. Multiple steps and multiple aspects within each step require optimization. In addition to workflow complexity and a lack of standardization, reproducible and fast protein digestion remains challenging, particularly to novice users.
To address these issues, scientists at Waters have developed ProteinWorks™ Digest Kits. With five options, these validated kits simplify the protein bioanalysis workflow by packaging together the reliable reagents, microelution solid-phase extraction tools, a collection module, and proven protocols that analysts need for successful sample preparation. The kits and their protocols are designed to reduce workflow steps and variables while providing reproducible, sensitive, and accurate results.
Learn more from our experts
One of the industry’s well-known experts on peptide and protein bioanalysis, Dr. Erin Chambers, discussed “Practical Considerations for LC/MS Bioanalysis of Proteins via the Surrogate Peptide Approach” at a recent industry conference. Hear her insights in this video.
Get the details in our application notes
References:
- https://www.antibodysociety.org/news/approved_mabs.php
- https://www.bptc.com/sites/default/files/articles/ecker-2015-the_therapeutic_monoclonal_antibody_market-rprnt.pdf
- https://www.labome.com/method/Quantitative-Bioanalysis-of-Proteins-by-Mass-Spectrometry.html
Popular Topics
ACQUITY QDa (16) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biosimilars (11) biotherapeutics (16) case study (16) chromatography (14) data integrity (21) food analysis (12) HPLC (15) LC-MS (21) liquid chromatography (LC) (19) mass detection (15) mass spectrometry (MS) (54) method development (13) STEM (12)